- SAR-bisPSMA Overview
- 67Cu-SAR-bisPSMA
- 64Cu-SAR-bisPSMA

Theranostic SECuRE trial
67Cu-SAR-bisPSMA is currently being studied in the SECuRE trial for patients with metastatic castrate resistant prostate cancer (mCRPC)
SECuRE is a US-based Phase I/IIa theranostic trial for identification and treatment of an advanced form of prostate cancer, mCRPC. It is a multi-centre, single arm, dose escalation study with a cohort expansion (NCT 04868604). Participants in the first three cohorts in the dose escalation phase of the trial have been treated with single doses of 4GBq, 8GBq and 12GBq doses of 67Cu SAR-bisPSMA and no dose limiting toxicities (DLTs) have been observed in these participants.
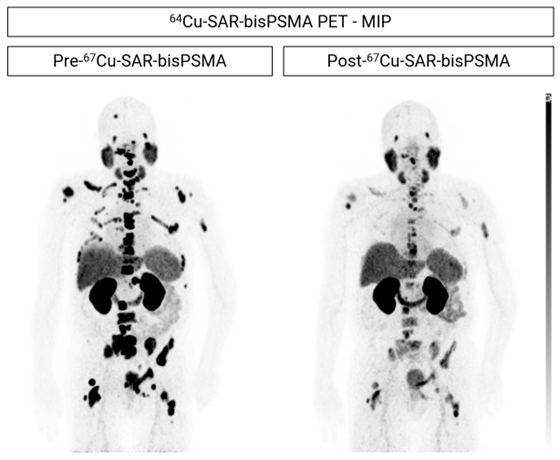
Figure 1. Participant from the SECuRE trial showing reduction in uptake of 64Cu-SAR-bisPSMA in prostate cancer lesions. The participant was treated with androgen deprivation therapy (ADT), androgen receptor pathway inhibition (ARPI) therapy, chemotherapy and 2 investigational agents prior to enrolling in the SECuRE study (PSA 270.9 ng/ml at study entry). The participant received a single dose of 67Cu-SAR-bisPSMA (12GBq), which led to the reduction in uptake of 64Cu-SAR-bisPSMA in the lesions. PSA reduction: 92.3%. Total body tumour reduction: SUVmax from 51.7 to 19.0 (63.2% reduction) and tumour volume from 1,040.9 to 635.4 ml (39.0% reduction). MIP.
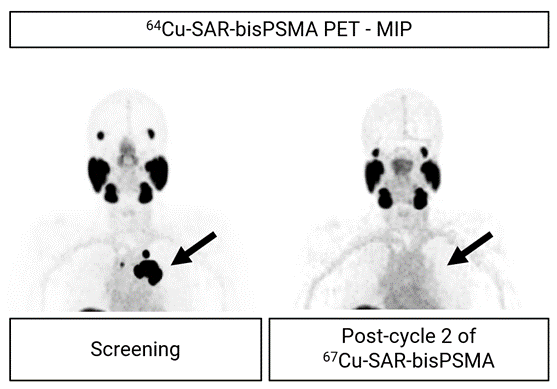
Figure 2. PET images showing uptake of 64Cu-SAR-bisPSMA in prostate cancer lesions at screening (arrow, left image; maximum standardised uptake value [SUVmax] 140.1). Image post-treatment show no 64Cu-SAR-bisPSMA uptake (arrow, right image). Images shown as maximum intensity projection.
Complete response with two doses of 8GBq of 67Cu-SAR-bisPSMA
The first patient ever to be dosed with two cycles of 67Cu-SAR-bisPSMA at 8GBq achieved a complete response to treatment based on RECIST criteria, had a prostate-specific antigen (PSA) reduction from 47.2 ng/mL to undetectable levels and had no lesion uptake using 64Cu-SAR-bisPSMA positron emission tomography (PET) imaging (Figure 2).
The patient received the first cycle of 67Cu-SAR-bisPSMA as part of cohort 2 of SECuRE and a second cycle under the US FDA EAP, as requested by the patient’s clinician. Adverse events related to 67Cu-SAR-bisPSMA included dry mouth, altered taste, thrombocytopenia (all Grade 1, improved), fatigue (Grade 2, resolved) and anaemia (Grade 3, improved to Grade 2). Prior to 67Cu-SAR-bisPSMA, the patient had failed multiple lines of treatment, including hormone therapy, an investigational agent and chemotherapy.
Learn more about this patient case study here.
