- SAR-bisPSMA Overview
- 67Cu-SAR-bisPSMA
- 64Cu-SAR-bisPSMA
64Cu-SAR-bisPSMA has demonstrated that it is safe and highly effective in detecting prostate cancer lesions in patients prior to initial definite therapy (PROPELLER trial, NCT 048393671) as well as in patients with biochemical recurrence (BCR) of prostate cancer (COBRA trial, NCT 05249127).

Diagnostic PROPELLER trial – Phase I
The PROPELLER trial in prostate cancer patients prior to radical-prostatectomy showed a 2-3x increase in uptake of 64Cu-SAR-bisPSMA in lesions compared to 68Ga-PSMA-11 (Figure 1). Learn more about the PROPELLER trial here.
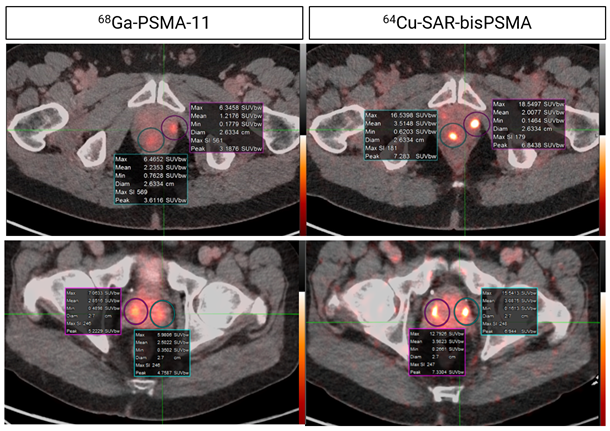
Figure 1. Concordant lesions on 64Cu-SAR-bisPSMA (200 MBq) and 68Ga-PSMA-11 positron emission tomography/computed tomography (PET/CT) consistently showed higher maximum standardised uptake values (SUVmax), mean standardised uptake values (SUVmean) and tumour-to-background ratios (TBR) with 64Cu-SAR-bisPSMA compared to 68Ga-PSMA-11 in all cohorts (statistically significant values for all parameters). Interval between scans: top 8 days; bottom 35 days. Images: PET/CT fusion.
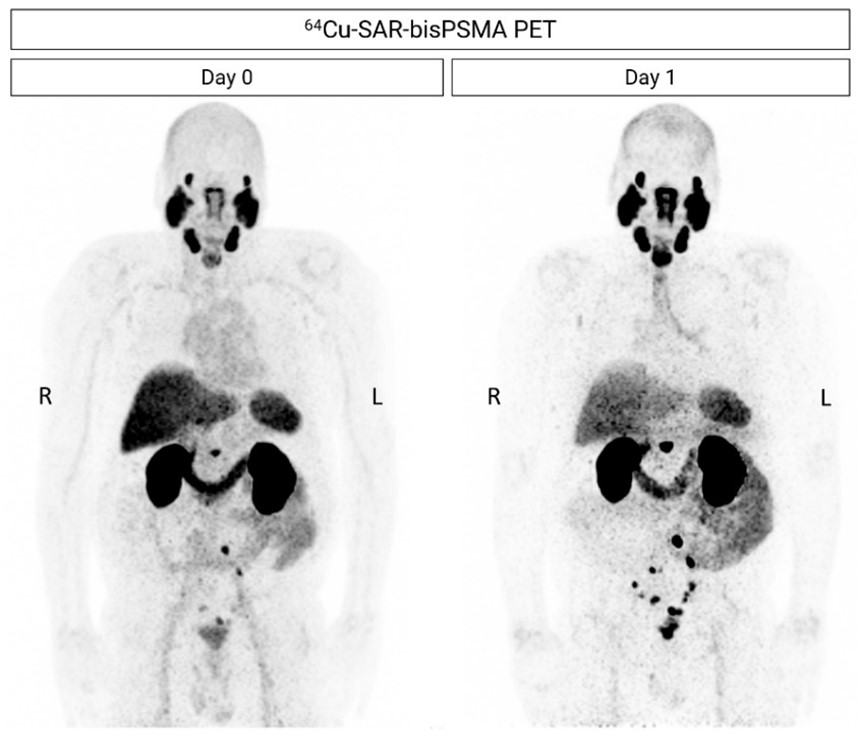
Figure 2. Next-day imaging identified additional lesions compared to same-day imaging. 64Cu-SAR-bisPSMA PET showing positive lymph nodes (LNs) in the pelvic, extra-pelvic (retroperitoneal) and prostatic bed regions. Images are displayed at Maximum Intensity Projection (MIP).

Diagnostic COBRA trial – Phase I/II
The Phase I/II COBRA trial in patients with BCR of prostate cancer imaged patients on the same-day (1-4 hours) and next-day (~24 hours). The number of lesions identified by 64Cu-SAR-bisPSMA on next-day imaging almost doubled compared to same-day imaging (Figure 2). Next-day imaging also identified lesions in the 2-millimetre range.
Learn more about the COBRA trial here.

Diagnostic CLARIFY trial – registrational Phase III
Based on positive results from the Phase I PROPELLER trial, Clarity has initiated the pivotal CLARIFY trial (NCT 06056830) to evaluate the safety and efficacy of 64Cu-SAR-bisPSMA in patients with confirmed prostate cancer prior to initial definitive treatment.
Learn more about the CLARIFY trial here.
