- SAR-bisPSMA Overview
- 67Cu-SAR-bisPSMA
- 64Cu-SAR-bisPSMA
64/67Cu-SAR-bisPSMA:
A Next-Generation PSMA Theranostic
SAR-bisPSMA is a next generation theranostic radiopharmaceutical with dual PSMA-targeting agents linked to Clarity’s SAR chelator technology. It utilises the isotopes of copper for imaging (64Cu or Cu-64) and therapy (67Cu or Cu-67).
Prostate cancer is the second most common cancer in men and the 5th leading cause of death in men worldwide. Patients diagnosed with prostate cancer at later stages have poor treatment outcomes, indicating a high unmet need for early detection and better treatment options for metastatic castration-resistant prostate cancer.
PSMA is a protein expressed in >87% of patients with prostate cancer and is an excellent diagnostic and therapeutic target.
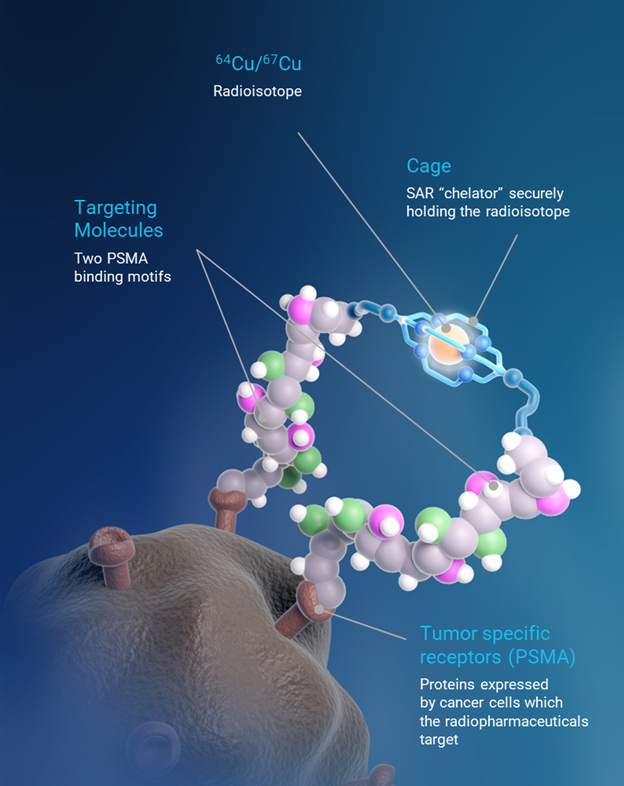
Clarity is running a therapy program in metastatic castrate resistant prostate cancer (mCRPC) with 64Cu-SAR-bisPSMA and 67Cu-SAR-bisPSMA and multiple diagnostic trials in line with advice received from the US Food and Drug Administration (FDA) to address the two relevant patient populations for registration of diagnostic 64Cu-SAR-bisPSMA:
- pre-prostatectomy/pre-definitive treatment of patients with confirmed prostate cancer; and
- patients with biochemical recurrence (BCR) of prostate cancer.
Pre-clinical data confirms best-in-class potential with optimised SAR-bisPSMA molecule
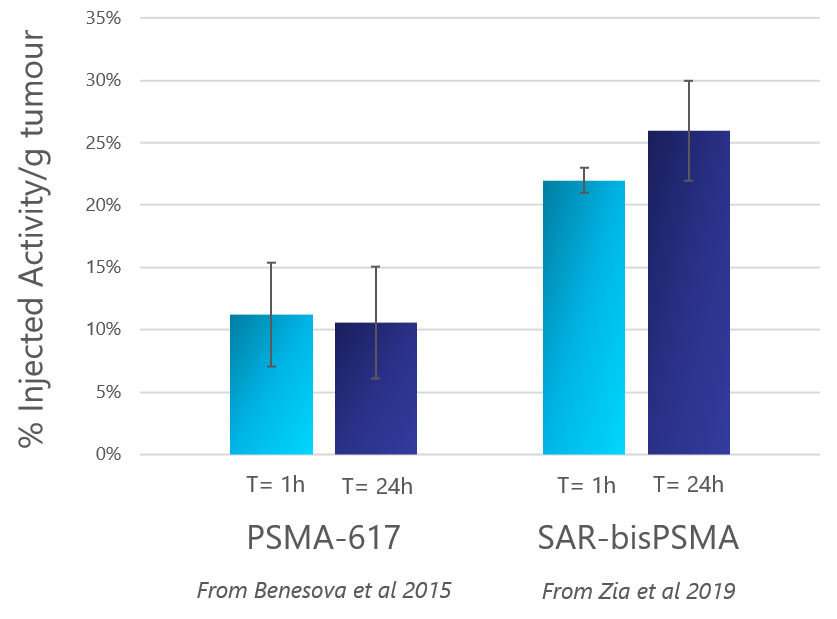
A comparison of the % injected activity in tumours in the same preclinical model (LNCaP) for PSMA-617 and SAR-bisPSMA demonstrates that SAR-bisPSMA is potentially the best-in-class theranostic agent for prostate cancer based on the superior uptake of SAR-bisPSMA in tumours at 1 and 24 hours compared to PSMA-617. This improvement is hypothesised to be driven by the two PSMA-targeting molecules in the optimised structure of SAR-bisPSMA.
Comparison of % Injected Activity/g in tumour of
SAR-bisPSMA to PSMA-617
SAR-bisPSMA also has the ideal product characteristics for a radiopharmaceutical: fast uptake and retention in tumours, rapid clearance from normal organs and significant anti-tumour effect in preclinical models.
64Cu-SAR-bisPSMA
67Cu-SAR-bisPSMA
Zia et al., 2019. Ang.Chem
Zia et al., 2019. Ang.Chem
McInnes et al., 2020. JNM
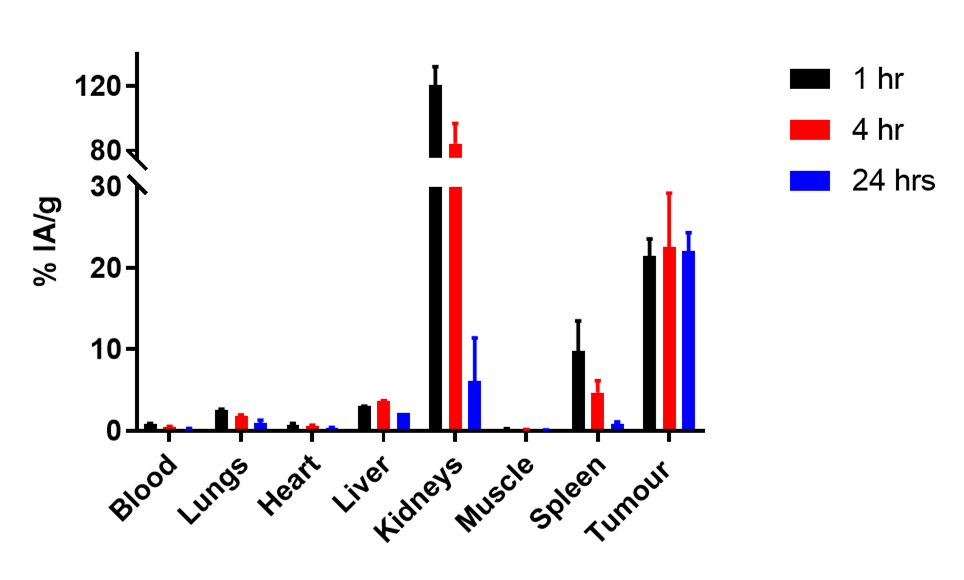
Fast uptake and retention in tumours
Biodistribution in a LNCaP xenograft model of 64Cu SAR-bisPSMA showing high uptake and retention in tumours as well as rapid clearance from key organs over 24 hours
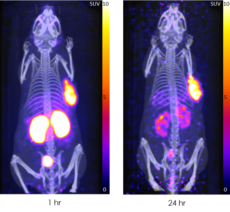
Rapid kidney clearance of unbound product
Positron Emission Tomography images showing 64Cu SAR-bisPSMA targeting to tumours within 1 hour and otherwise rapid kidney clearance
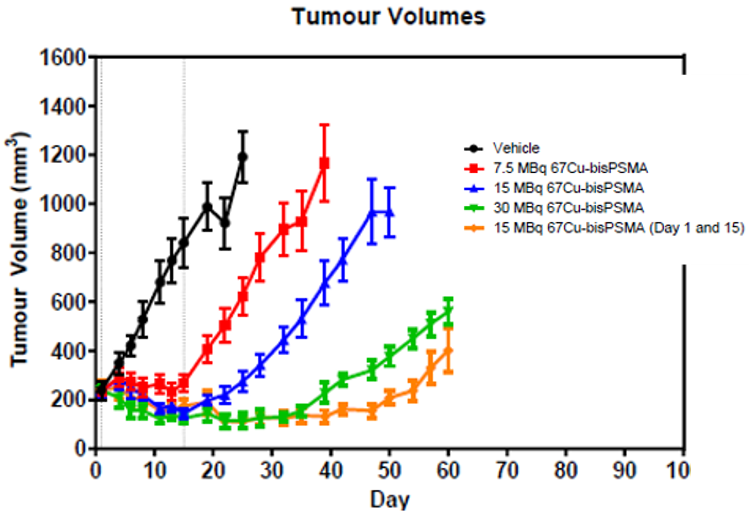
Significant anti-tumour effect
Xenograft mice with LNCaP tumours were injected with a single or repeat dose of up to 30 MBq of 67Cu SAR-bisPSMA.
Multiple clinical trials are generating robust data on SAR-bisPSMA
Study name | Products | Indication | Phase | Number of Patients | Location | Status | Reference |
 | 64SAR-bisPSMA/ 67SAR-bisPSMA | Prostate therapy | I/IIa | 34 | USA | Recruiting | |
 | 64Cu SAR-bisPSMA | Prostate diagnostic | III | 383 | USA/AUS | Recruiting | |
64Cu SAR-bisPSMA | Prostate diagnostic | I/II | 50 | USA | Study objectives reached | ||
 | 64Cu SAR-bisPSMA | Prostate diagnostic | I | 30 | AUS | Study objectives reached |

