Clinical Development Pipeline
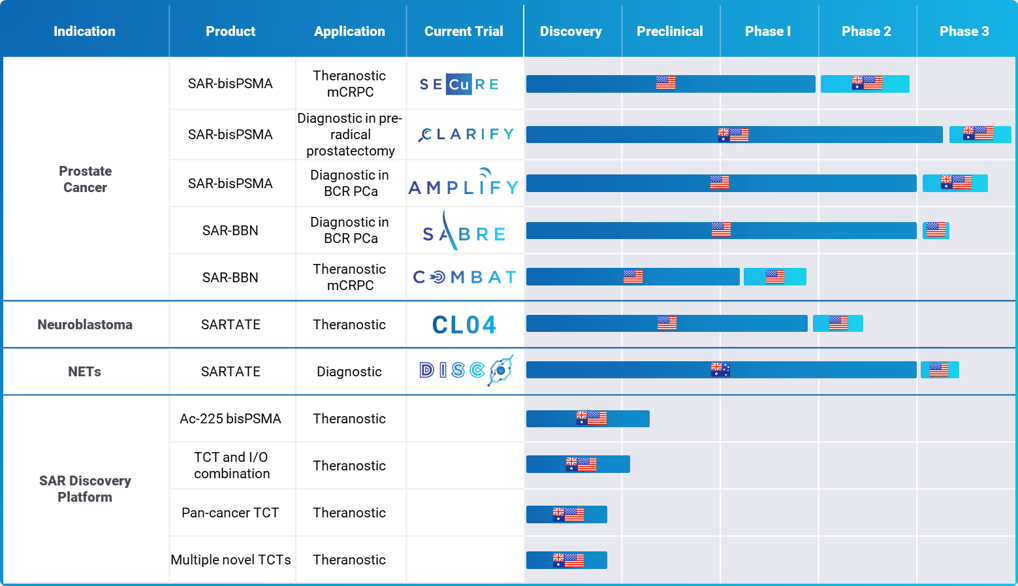
*All US studies are conducted under IND
**As of November 2024
***Note clinical development pipeline is indicative only and is subject to review
Products in Development
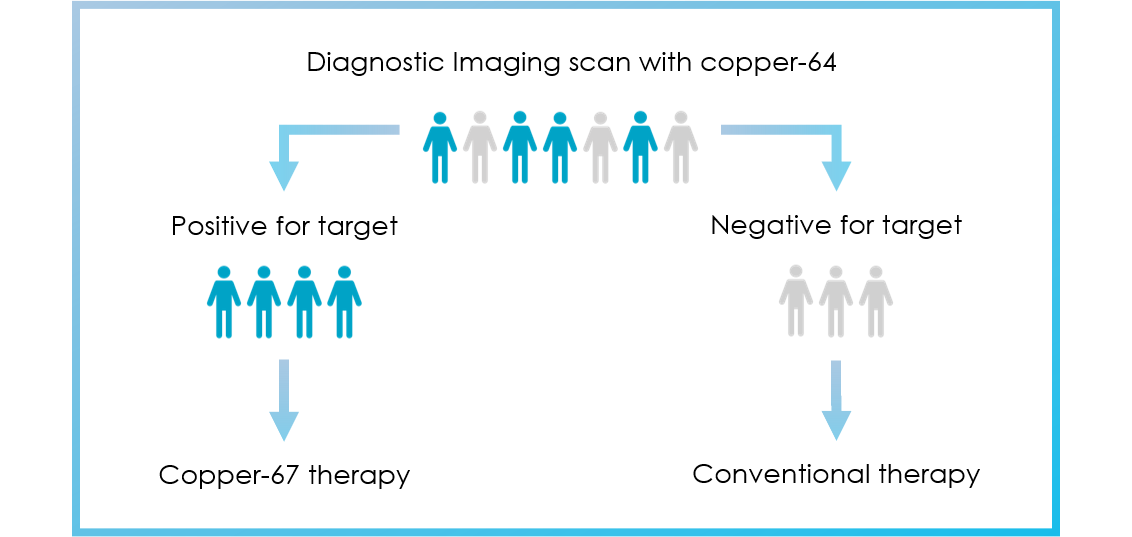
Clarity’s theranostic programs aim to increase the probability of treatment success by selecting patients that demonstrate uptake of the diagnostic agents to visualise their cancer prior to therapy.
For indications such as prostate cancer and neuroendocrine tumours (NETs), the diagnostic products based on copper-64 (Cu-64) are also in development as stand-alone products to diagnose and manage disease in a broader spectrum of patients.
Clarity’s clinical pipeline is focused on theranostic product development for the following indications:
- Prostate cancer therapy (64/67Cu-SAR-bisPSMA and 64/67Cu-SAR-Bombesin)
- Neuroblastoma therapy (SARTATE)
Diagnostic studies are also underway in:
- Prostate cancer (64Cu-SAR-bisPSMA and 64Cu-SAR-Bombesin)
- Neuroendocrine tumours (64Cu-SARTATE)