Sydney, Australia 5 March 2025
Highlights
- Clarity has signed a new agreement with The University of Queensland (UQ) advanced imaging at the Australian Institute for Bioengineering and Nanotechnology (AIBN) for supply of copper-64 (Cu-64 or 64Cu) isotope.
- The copper-64 supply will be used to expand the manufacturing capability of the diagnostic 64Cu-SAR-bisPSMA product for the Phase III CLARIFY and AMPLIFY registrational trials in Australia, the ongoing investigator-initiated trial (IIT), Co-PSMA, at St Vincent’s Hospital, Sydney, as well as the SECuRE theranostic trial in Australia.
- In addition to the ongoing clinical trials, the copper-64 supply will also support the advancement of Clarity’s pre-clinical program, including SAR-bisFAP and SAR-trastuzumab theranostic programs.
- The AIBN houses an on-site cyclotron and state-of-the-art radiochemistry facilities which will be supplying copper-64 every week to Australian sites and will complement existing copper-64 supply
- This agreement enhances Clarity’s ongoing collaborations with UQ, which include a number of research and development initiatives, including being an active participant in the Australian Research Council (ARC) Hub for Advanced Manufacture of Targeted Radiopharmaceuticals (AMTAR).
Clarity Pharmaceuticals (ASX: CU6) (“Clarity” or “Company”), a clinical-stage radiopharmaceutical company with a mission to develop next-generation products that improve treatment outcomes for children and adults with cancer, is pleased to announce the signing of a new Supply Agreement for copper-64 with UQ AIBN.
The UQ AIBN Supply Agreement of copper-64 will support clinical trials with 64Cu-SAR-bisPSMA in Australia, offering this promising imaging agent to prostate cancer patients in need of novel diagnostic options. Additionally, the Agreement will support the rollout of 2 theranostic pre-clinical programs, 64/67Cu-SAR-bisFAP and 64/67Cu-SAR- trastuzumab.
With multiple clinical trials with 64Cu-SAR-bisPSMA, such as the ongoing registrational CLARIFY trial, Phase I/IIa theranostic SECuRE trial and the upcoming registrational Phase III AMPLIFY trial, as well as the Co-PSMA IIT, led by Prof Louise Emmett at St Vincent’s Hospital Sydney, the Agreement will provide additional capacity of copper-64 to ensure abundant and seamless supply of the isotope. The recent receipt of 2 Fast Track Designations (FTDs) by the US Food and Drug Administration (FDA) for the diagnostic 64Cu-SAR-bisPSMA product1,2 will enable Clarity to accelerate the development of its program with this optimised agent in the pre-prostatectomy setting as well as in patients with biochemical recurrence (BCR) of prostate cancer. The granting of these FTDs is indicative of the high unmet need in prostate cancer imaging despite the use of first-generation PSMA products in the market and is testament to the high quality of science and data Clarity is generating with this product.
64Cu-SAR-bisPSMA differentiates itself from the current generation of radio-diagnostics in 2 distinct ways. Firstly, the dimer “bis” molecule enables increased uptake and retention of the imaging agent in tumours3,4. Similar to Clarity’s platform SAR Technology, it has been developed in Australia, at the benchtop of Australian science, with the intent of overcoming the shortfalls of the current generation of prostate-specific membrane antigen (PSMA) targeting products. The clinical data that Clarity has generated to date confirms these results initially seen in preclinical development3,4. The second advantage is associated with the benefits of the copper-64 isotope, which has a half-life much longer (12.7 hours) than that of gallium-68 and fluorine-18 (less than 2 hours). This enables next-day imaging, a feature not available with the current-generation radio-diagnostics. Clarity’s clinical trials with 64Cu-SAR-bisPSMA to date have demonstrated a number of benefits of next-day imaging. In the Phase II COBRA study, next-day 64Cu-SAR-bisPSMA imaging enabled detection of lesions in up to 80% of participants in comparison to up to 58% on the same-day imaging, and the number of lesions detected on the next-day scans almost doubled in comparison to the same-day scans5.
In addition to the clinical benefits, the longer half-life of copper-64 enables manufacturing and logistical advantages. While the current-generation of radio-diagnostics require an expensive and extensive network of cyclotrons, radioisotope generators and radiopharmacies next to imaging sites due to their short half-life, resulting in a short shelf-life, copper-64 can be centrally produced in large volumes on existing cyclotrons. Copper-64 based diagnostics can then be manufactured and shipped as finished drug products to any treatment facility in the country, overcoming the many issues with product shortage and expiry, patient scheduling and limited geographic distribution of the current generation of products. Products like PYLARIFY® are already hitting supply limits in the US due to limited cyclotron availability and the competing use of fluorine-18, which is prioritised for fluorodeoxiglucose (FDG). In contrast, copper-64 can be made on the same cyclotrons as fluorine-18, such as that at UQ AIBN, but at different times to fluorine-18, which has to be manufactured strictly in the morning in order to deliver it to patients before the half-life expires.
Clarity’s Executive Chairperson, Dr Alan Taylor, commented, “We are pleased to continue building a successful Australian life-sciences story and benefiting Australian patients and their treating clinicians. We are proud to have been able to progress our technology from the benchtop at some of the most prominent institutes in Australia to Phase III trials at leading clinical sites in Australia and the US. This agreement with the UQ AIBN not only builds on years of close ties between Clarity and the Australian scientific and clinical communities but also reflects our strong focus on continued partnership and synergies that can be derived from these important collaborations. We are dedicated to continuing to work with the leading research and development (R&D) organisations in Australia and giving back to the scientific and clinical community in our country in order to get closer to our ultimate goal of improving treatment outcomes for people with cancer.
“UQ advanced imaging has been a long-standing collaborator of Clarity through a number of R&D initiatives, including being an active participant in the ARC Hub for AMTAR. We have experienced first-hand the strong scientific focus, drive for innovation and the collaborative community and look forward to continuing working together to bring novel products to patients in need of better diagnostic and treatment options.
“The AIBN houses an on-site cyclotron and state-of-the-art radiochemistry facilities, supplying copper-64 every week. Securing a strong supply chain is pivotal as we ramp up our clinical trials and look to generate sufficient data for the approval of 64Cu-SAR-bisPSMA. This centralised distribution strategy with the AIBN supplying copper-64 for the manufacturing of our innovative 64Cu-SAR-bisPSMA diagnostic agent will ensure that patients and clinicians have secure and seamless access to the product as we continue to generate exciting data in our trials.
“We are also committed to exploring new ways in which we can leverage our unique advantages in the development of Targeted Copper Theranostics (TCTs) to bring new products for indications with high unmet needs. As such, the Supply Agreement will also support the recently announced development of SAR-bisFAP and SAR-trastuzumab theranostic programs.”
About SAR-bisPSMA
SAR-bisPSMA derives its name from the word “bis”, which reflects a novel approach of connecting two PSMA-targeting agents to Clarity’s proprietary sarcophagine (SAR) technology that securely holds copper isotopes inside a cage-like structure, called a chelator. Unlike other commercially available chelators, the SAR technology prevents copper leakage into the body. SAR-bisPSMA is a Targeted Copper Theranostic (TCT) that can be used with isotopes of copper-64 (Cu-64 or 64Cu) for imaging and copper-67 (Cu-67 or 67Cu) for therapy.
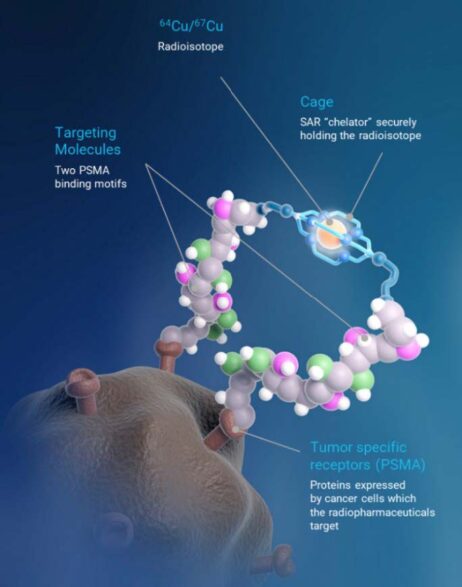
67Cu-SAR-bisPSMA and 64Cu-SAR-bisPSMA are unregistered products. The safety and efficacy of 67Cu-SAR-bisPSMA and 64Cu-SAR-bisPSMA have not been assessed by health authorities such as the US FDA or the Therapeutic Goods Administration (TGA). There is no guarantee that these products will become commercially available.
About Clarity Pharmaceuticals
Clarity is a clinical stage radiopharmaceutical company focused on the treatment of serious diseases. The Company is a leader in innovative radiopharmaceuticals, developing Targeted Copper Theranostics based on its SAR Technology Platform for the treatment of cancers in children and adults.
About UQ AIBN
Advanced imaging at AIBN brings together the skills of a critical mass of researchers and ‘state-of-the-art’ research imaging instruments. It is the only facility of its type in Australia, one of only a handful in the world. The 5,500 m2, $55M building was funded by the Federal Education Investment Fund in 2010 and contains over $50M of imaging and spectroscopy equipment, putting UQ’s researchers at the forefront of a field that is advancing swiftly.
Their researchers work on innovations in spectroscopic and imaging technology, imaging biomarker development and in biomedical research disciplines, frequently in collaboration with clinical research sites and other local, national, and international research institutes
Media Contacts
Clarity Pharmaceuticals
Dr Alan Taylor
Executive Chairperson
ataylor@claritypharm.com
Sodali
Catherine Strong
Investor/Media Relations
+61 (0)406 759 268
catherine.strong@sodali.com
References
- Clarity Pharmaceuticals. Clarity receives FDA Fast Track Designation for 64Cu-SAR-bisPSMA. https://www.claritypharmaceuticals.com/news/fast-track/
- Clarity Pharmaceuticals. Clarity receives U.S. FDA Fast Track Designation for 64Cu-SAR-bisPSMA in biochemical recurrence of prostate cancer. https://www.claritypharmaceuticals.com/news/ftd-2/
- Zia et al. A Bivalent Inhibitor of Prostate Specific Membrane Antigen Radiolabeled with Copper-64 with High Tumor Uptake and Retention. Angew Chem Int Ed Engl, 2019.
- Lengyelova et al. 64Cu-SAR-bisPSMA (PROPELLER) positron emission tomography (PET) imaging in patients with confirmed prostate cancer. ASCO, 2023.
- Nordquist et al. COBRA: Assessment of safety and efficacy of 64Cu-SAR-bisPSMA in patients with biochemical recurrence of prostate cancer following definitive therapy. EANM, 2024.
This announcement has been authorised for release by the Executive Chairperson.
