Sydney, Australia 17 July 2023
Clarity Pharmaceuticals (ASX: CU6) (“Clarity”, “the Company”), a clinical stage radiopharmaceutical company with a mission to develop next-generation products that improve treatment outcomes for children and adults with cancer, has been awarded First Place in the Oncology, Clinical Therapy & Diagnosis category at the world’s most prestigious nuclear medicine conference, Society of Nuclear Medicine and Molecular Imaging (SNMMI) 2023 Annual Meeting.
SNMMI is the premier educational, scientific, research and networking meeting in nuclear medicine and molecular imaging, showcasing the latest research and developments in the field.
The award relates to Clarity’s poster presentation detailing the results from the completed PROPELLER diagnostic trial, which showed that Clarity’s optimised 64Cu SAR-bisPSMA product was safe and effective for detecting PSMA-expressing lesions in men with prostate cancer.
Clarity’s Executive Chairperson, Dr Alan Taylor, commented, “We are excited to be awarded First Place for our poster at SNMMI. The PROPELLER trial was our first opportunity to showcase how our PSMA agent is different to the first generation PSMA diagnostic agents, and it is truly an honour to receive such prominent recognition of the quality and significance of our PROPELLER trial results.
“Our PROPELLER trial showed that 64Cu SAR-bisPSMA had a high level of uptake in lesions, enabling the detection of smaller lesions and in some cases additional lesions that would otherwise have gone undetected, compared to the standard of care imaging product, 68Ga PSMA-11, a generic product that has been commercialised by University of California, Los Angeles (UCLA) academics, Novartis and Telix. The lesions showed 2-3 times higher uptake of 64Cu SAR-bisPSMA compared to 68Ga PSMA-11 and were brighter on the scans.
“In the pre-prostatectomy patient population, it is critically important to detect disease which has spread outside the prostate. This is because the side effects of prostatectomy are significant, and for patients with lesions outside of the prostate other treatment options may be considered. Given the higher uptake of our product in lesions, we were able to detect more lesions using 64Cu SAR-bisPSMA compared to 68Ga PSMA-11, which in future may enable better treatment options and quality of life for these patients.”
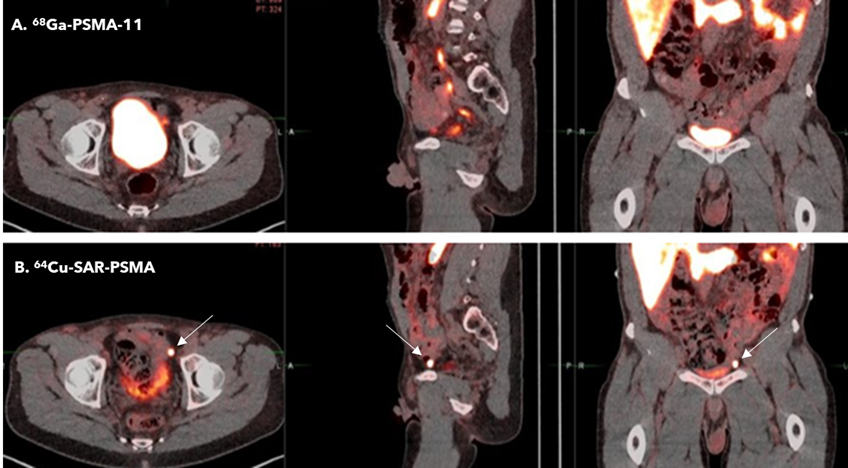
Figure 1. PET/CT demonstrated uptake of 64Cu SAR-bisPSMA at 200MBq (B) in a left pelvic lymph node according to both readers and prostate cancer was confirmed via histopathology. Readers did not detect uptake in pelvic lymph nodes on the 68Ga-PSMA-11 PET/CT (A). Arrows highlight the additional node. Interval between serial imaging: 7 days.
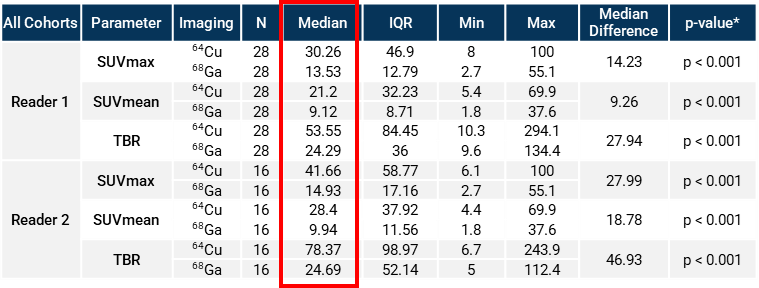
Figure 2. *Comparison of imaging methods undertaken with two-sided Wilcoxon signed-rank test. The table shows 64Cu SAR-bisPSMA was 2-3 times higher for SUVmax, SUVmean and tumour to background ratio, compared to 68Ga PSMA-11.
“The compelling results from PROPELLER set the scene for our new Phase 3 trial with 64Cu SAR-bisPSMA which will commence in late 2023,” said Dr Taylor.
Earlier this month, Clarity completed an end of phase meeting with the FDA and are now progressing towards the commencement of a pivotal Phase 3 trial, named CLARIFY, for 64Cu SAR-bisPSMA as a diagnostic agent in prostate cancer.
“CLARIFY is a pivotal trial, which means the final study results should provide enough evidence to apply to the FDA for approval of 64Cu SAR-bisPSMA as a new diagnostic imaging agent in prostate cancer. We’re very excited to be taking this big step forward,” Dr Taylor added.
A total of 383 patients with newly diagnosed prostate cancer will take part in the CLARIFY study across multiple clinical sites.
“We continue to build on the diagnostic and therapeutic evidence of our SAR-bisPSMA product to provide patients with better options to diagnose and treat their prostate cancer, and we look forward to providing further updates on all of our trials with this agent very soon,” Dr Taylor concluded.
About SAR-bisPSMA
SAR-bisPSMA derives its name from the word “bis”, which reflects a novel approach of connecting two PSMA binding motifs to Clarity’s proprietary sarcophagene (SAR) technology that securely holds copper isotopes inside a cage-like structure, called a chelator. Unlike other commercially available chelators, the SAR technology prevents copper leakage into the body. SAR-bisPSMA is a TCT that can be used with isotopes of copper-64 (Cu-64 or 64Cu) for imaging and copper-67 (Cu-67 or 67Cu) for therapy.
64Cu SAR-bisPSMA and 67Cu SAR-bisPSMA are investigational products and not yet approved by health authorities.
About Prostate Cancer
Prostate cancer is the second most common cancer diagnosed in men globally and the fifth leading cause of cancer death worldwide. The American Cancer Institute estimates in 2023 there will be 288,300 new cases of PC in the US and around 34,700 deaths from the disease.
About Clarity Pharmaceuticals
Clarity is a clinical stage radiopharmaceutical company focused on the treatment of serious disease. The Company is a leader in innovative radiopharmaceuticals, developing TCTs based on its SAR technology platform for the treatment of children and adults with cancer.
Media Contacts
Clarity Pharmaceuticals
Dr Alan Taylor
Executive Chairman
+61 (0)413 871 165
ataylor@claritypharm.com
Citadel-MAGNUS
Catherine Strong
Investor/Media Relations
+61 (0)406 759 268
cstrong@citadelmagnus.com
This announcement has been authorised for release by the Executive Chairman.
