Sydney, Australia 10 August 2023
Highlights
- Cohort 2 of the theranostic SECuRE trial investigating 64Cu/67Cu SAR-bisPSMA in metastatic castrate-resistant prostate cancer (mCRPC) has been completed in 3 participants who received therapy with 67Cu SAR-bisPSMA at the dose level of 8GBq.
- No dose limiting toxicities (DLTs) have been reported in cohort 2.
- The Safety Review Committee (SRC) has recommended that the trial continues to cohort 3.
- Data from cohort 2 indicates positive effects of the 8GBq dose of 67Cu SAR-bisPSMA on all patients, demonstrated by a remarkable reduction in Prostate Specific Antigen (PSA) levels within weeks of a single dose.
- Additional therapy cycles of 67Cu SAR-bisPSMA have been requested by clinicians under the United States Food and Drug Administration (US FDA) Expanded Access Program (EAP).
- Under the EAP, a participant from cohort 1 who exhibited a greater than 50% reduction in PSA after one administration of the lowest dose level of 4GBq of 67Cu SAR-bisPSMA has now completed a total of four doses at the same dose level with a greater than 90% drop in PSA and decreased intensity of Cu-67 uptake on SPECT-CT images.
- Recruitment has opened at clinical sites in the US at the cohort 3 dose level of 12GBq of 67Cu SAR-bisPSMA, the highest dose cohort in the dose escalation phase, and patients are currently in screening for all available slots.
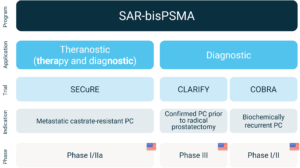
Clarity Pharmaceuticals (ASX: CU6) (“Clarity”, “the Company”), a clinical stage radiopharmaceutical company with a mission to develop next-generation products that improve treatment outcomes for children and adults with cancer, is pleased to announce the successful completion of cohort 2 and advancement to cohort 3 in the dose escalation phase of its Phase I/II theranostic trial, SECuRE, evaluating 64Cu/67Cu SAR-bisPSMA in patients with mCRPC.
The SECuRE trial (NCT04868604)1 is a Phase I/IIa theranostic trial for identification and treatment of Prostate-Specific Membrane Antigen (PSMA) expressing mCRPC using 64Cu/67Cu SAR-bisPSMA. 64Cu SAR-bisPSMA is used to visualise PSMA expressing lesions and select candidates for subsequent 67Cu SAR-bisPSMA therapy. The trial is a multi-centre, single arm, dose escalation trial with a cohort expansion involving up to 44 patients in the US. The aim of the trial is to determine the safety and efficacy of 67Cu SAR-bisPSMA for the treatment of prostate cancer.
The second cohort of the dose escalation, where 3 participants received a single administration of 8GBq of 67Cu SAR-bisPSMA, has been completed. No DLTs have been reported in any of the patients dosed to date. The SRC, responsible for assessing safety of participants and overseeing the general progress of the trial, has assessed the data and recommended progressing the trial to cohort 3, increasing the dose to 12GBq. The third cohort will be the last to assess single doses of 67Cu SAR-bisPSMA and will be followed by a multi-dose cohort, pending safety evaluation. The 3 participants in cohort 2 have been monitored by their physicians for safety and treatment response as per the trial protocol. All 3 participants in cohort 2 remain on the trial following their recent administration of 8GBq of 67Cu SAR-bisPSMA and are demonstrating a PSA reduction, with 2 of the 3 participants exhibiting an initial PSA reduction of ~90%. A PSA decline of 50% or greater is one of the primary endpoints of the SECuRE trial and a commonly used surrogate endpoint for efficacy in this patient population.
Dr Luke Nordquist, CEO, Urologic Medical Oncologist and Principal Investigator at the Urology Cancer Center / XCancer Omaha, NE, commented, “We are excited by the remarkable PSA declines seen in all three patients in cohort 2 with just a single dose of 8GBq of 67Cu SAR-bisPSMA. I have not observed PSA responses like this after a single dose of any agent and, considering the excellent safety profile we have seen to date in the first two cohorts of this study, we really look forward to progressing the development of this promising therapy. While in the VISION trial2 with 177Lu PSMA-617 we did see a >80% reduction in PSA in roughly 33% of patients, this was after up to six 7.4GBq doses of 177Lu PSMA-617 spaced out over a period of up to 30 weeks. If a single 8GBq dose of 67Cu SAR-bisPSMA can deliver so much benefit to the patients, we are excited to see how a single 12GBq dose will benefit patients in cohort 3 and to explore the effect of multiple dosing. If similar responses can be replicated in larger patient numbers, 67Cu SAR-bisPSMA may become the gold standard therapeutic agent for patients with mCRPC once approved.”
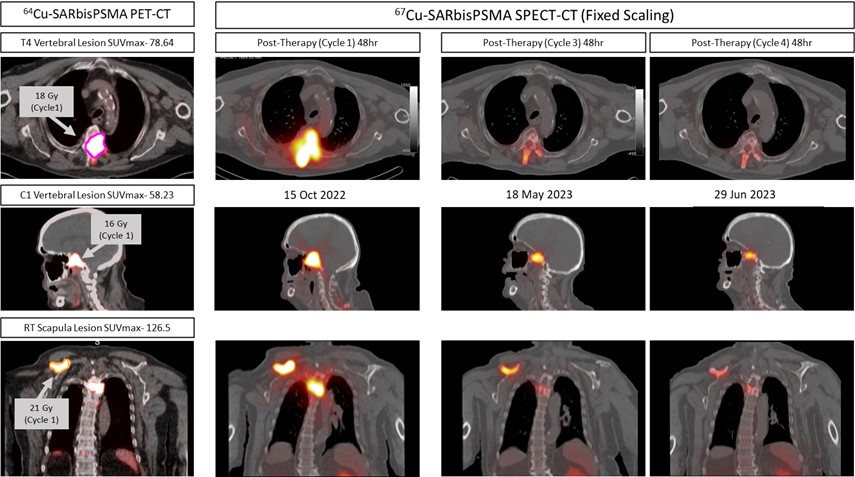
Additional therapy cycles of 67Cu SAR-bisPSMA have been requested by clinicians under the FDA EAP for patients who participated in the SECuRE trial. SPECT-CT images depicted below were collected 48 hours after the first, third and fourth administrations of 4GBq of 67Cu SAR-bisPSMA in a patient from cohort 1 who received additional cycles under the EAP.
SPECT-CT images collected following the third and fourth therapy cycle demonstrate a reduction in the intensity of therapeutic 67Cu SAR-bisPSMA product uptake at the tumour sites. A reduction of greater than 50% in PSA levels was observed in this patient following the first administration of 4GBq of therapeutic 67Cu-SAR-bisPSMA and a drop of greater than 90% in PSA was observed after the fourth administration of 4GBq of 67Cu-SAR-bisPSMA.
Clarity’s Executive Chairperson, Dr Alan Taylor, commented, “We are very excited to observe such a dramatic response in prostate cancer patients from cohort 2 following a single dose of 8GBq of 67Cu SAR-bisPSMA. SAR-bisPSMA aims to be a best-in-class PSMA product due to its differentiation from all other PSMA-targeted products in the market and in development that only have a single PSMA-targeting agent. We purposely designed and optimised SAR-bisPSMA to have two PSMA-targeting agents to address the challenges of low uptake and retention that the first generation of PSMA products suffer from. In pre-clinical and clinical development to date, we have observed two to three times the uptake of SAR-bisPSMA in tumours, followed by retention in tumours out to at least 96 hours. Although our data is early, the higher uptake and retention of product, coupled with the advantageous properties of copper-67, has shown quite impressive responses from single doses and we look forward to exploring the clinical benefits of 67Cu SAR-bisPSMA at the higher 12GBq level and over multiple treatment cycles. With commercial quantities of the 67Cu radioisotope now being routinely produced domestically in the US by our exclusive supplier, NorthStar, we see a clear path to commercialisation as we continue to push forward through clinical trials for 67Cu SAR-bisPSMA and bringing this product to the greater prostate cancer patient population.
“Prostate cancer is one of the largest oncology indications worldwide and, based on our estimates, represents a US$5-10 billion therapy market for PSMA targeting radiopharmaceuticals. Radiopharmaceuticals are expected to play an increasingly important role in the management of patients with prostate cancer, however, challenges associated with the current generation of products prevail. Clarity’s Targeted Copper Theranostic (TCT) platform represents the next-generation platform in radiopharmaceuticals to improve treatment outcomes for children and adults with cancer as well as resolve the supply and manufacturing issues associated with the first generation of products. Because of these characteristics, TCTs are ideally positioned to enable the field to expand into the oncology market, addressing large indications such as prostate cancer and beyond.
“We look forward to sharing more data on 67Cu SAR-bisPSMA as the SECuRE trial continues to progress and any further updates from patients who may receive single or multiple doses of 67Cu SAR-bisPSMA in our programs,” said Dr Taylor.
Overview of Clarity’s SAR-bisPSMA clinical program
About SAR-bisPSMA
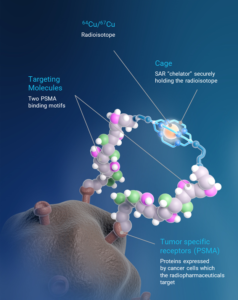
SAR-bisPSMA derives its name from the word “bis”, which reflects a novel approach of connecting two PSMA-targeting agents to Clarity’s proprietary sarcophagine (SAR) technology that securely holds copper isotopes inside a cage-like structure, called a chelator. Unlike other commercially available chelators, the SAR technology prevents copper leakage into the body. SAR-bisPSMA is a TCT that can be used with isotopes of copper-64 (Cu-64 or 64Cu) for imaging and copper-67 (Cu-67 or 67Cu) for therapy.
64Cu SAR-bisPSMA and 67Cu SAR-bisPSMA are unregistered products. Individual results may not represent the overall safety and efficacy of the products. The data outlined in this announcement has not been assessed by health authorities such as the US Food and Drug Administration (FDA). A clinical development program is currently underway to assess the efficacy and safety of these products. There is no guarantee that these products will become commercially available.
About Prostate Cancer
Prostate cancer is the second most common cancer diagnosed in men globally and the fifth leading cause of cancer death worldwide3. The American Cancer Institute estimates in 2023 there will be 288,300 new cases of prostate cancer in the US and around 34,700 deaths from the disease4.
About Clarity Pharmaceuticals
Clarity is a clinical stage radiopharmaceutical company focused on the treatment of serious disease. The Company is a leader in innovative radiopharmaceuticals, developing TCTs based on its SAR technology platform for the treatment of children and adults with cancer.
www.claritypharmaceuticals.com
References
- ClinicalTrials.gov Identifier: NCT04868604, https://clinicaltrials.gov/ct2/show/NCT04868604
- ClinicalTrials.gov Identifier: NCT03511664, https://clinicaltrials.gov/ct2/show/NCT03511664
- Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries, https://acsjournals.onlinelibrary.wiley.com/doi/10.3322/caac.21660
- American Cancer Society: Key Statistics for Prostate Cancer, https://www.cancer.org/cancer/prostate-cancer/about/key-statistics.html
Media Contacts
Clarity Pharmaceuticals
Dr Alan Taylor
Executive Chairman
+61 (0)413 871 165
ataylor@claritypharm.com
Citadel-MAGNUS
Catherine Strong
Investor/Media Relations
+61 (0)406 759 268
cstrong@citadelmagnus.com
This announcement has been authorised for release by the Executive Chairman.