Sydney, Australia 22 March 2024
Highlights
- First participant in cohort 4 (first multi-dose cohort) in the theranostic SECuRE trial has been treated with 67Cu-SAR-bisPSMA at 12GBq.
- The SECuRE trial is investigating 64Cu/67Cu-SAR-bisPSMA in metastatic castrate-resistant prostate cancer (mCRPC) patients. Fifteen patients have been treated so far at 3 different dose levels of 67Cu-SAR-bisPSMA (single cycle): 4, 8 and 12GBq.
- Preliminary data shows that 67Cu-SAR-bisPSMA has a favorable safety profile and no dose limiting toxicities (DLTs) have been reported in any of the trial participants to date.
- Three participants will be enrolled in cohort 4 and will receive two cycles of 67Cu-SAR-bisPSMA (12GBq) with the potential to receive two additional doses under the trial (up to 4 doses in total). Three additional patients will then be enrolled, pending assessment by the Safety Review Committee (SRC) after the first two cycles.
- Cohort 4 is the last cohort of the dose escalation phase of SECuRE, which will be followed by a dose expansion phase (14 patients).
- Recruitment is ongoing at clinical sites in the US. All available patient slots for the first part of cohort 4 have been allocated and screening activities are ongoing.
Clarity Pharmaceuticals (ASX: CU6) (“Clarity”, “the Company”), a clinical stage radiopharmaceutical company with a mission to develop next-generation products that improve treatment outcomes for children and adults with cancer, is pleased to announce the dosing of the first participant with the first of two cycles of 12GBq of 67Cu-SAR-bisPSMA in cohort 4, the final cohort in the dose escalation phase of the SECuRE trial.
The SECuRE trial (NCT04868604)1 is a Phase I/IIa theranostic trial for identification and treatment of participants with Prostate-Specific Membrane Antigen (PSMA)-expressing mCRPC using 64Cu/67Cu SAR-bisPSMA. 64Cu SAR-bisPSMA is used to visualise PSMA-expressing lesions and select candidates for subsequent 67Cu-SAR-bisPSMA therapy. The trial is a multi-centre, single arm, dose escalation trial with a cohort expansion involving up to 44 participants in the US. The overall aim of the trial is to determine the safety and efficacy of 67Cu-SAR-bisPSMA for the treatment of prostate cancer.
Cohort 4 explores the anti-cancer effects of multiple therapy cycles of 67Cu-SAR-bisPSMA at the highest dose of 12GBq on the SECuRE trial participants. The fourth cohort is the last cohort of the dose escalation phase before moving into the Phase II stage of the trial with dose expansion in 14 participants, pending safety evaluation.
Cohort 4 is designed as a “3+3” cohort, where the first 3 participants will receive 2 therapy cycles followed by an SRC meeting before commencing recruitment of the final 3 participants. Based on the safety profile observed in the first 3 cohorts of the SECuRE trial, a change to the dosing schedule of cohort 4 from “2 doses” to “up to 4 doses” has been approved by the SRC. The amendment to the protocol is currently underway, which will be submitted to the US Food and Drug Administration (FDA) and respective Institutional Review Boards for implementation. This will allow patients who are benefiting from 67Cu-SAR-bisPSMA to receive 2 additional doses under the SECuRE trial in cohort 4 (up to 4 doses in total).
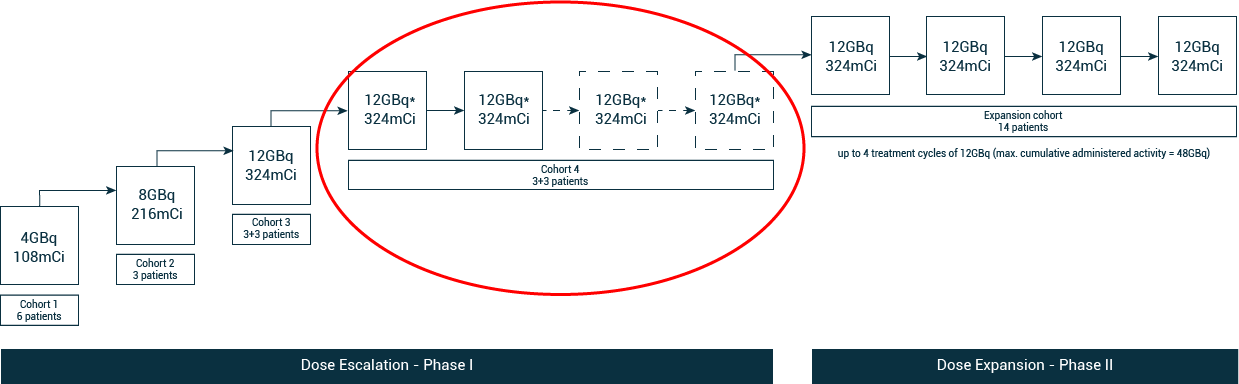
Figure 1. SECuRE Study Design.
*Patients in cohort 4 will receive 2 doses of 67Cu-SAR-bisPSMA (12GBq) according to the current study protocol. A protocol amendment is underway to allow 2 additional doses of 67Cu-SAR-bisPSMA in cohort 4. An SRC meeting will take place after participants receive their 2 doses, with a period of 6 weeks for safety follow-up.
Participants treated in the SECuRE trial to date have received multiple lines of therapy prior to their recruitment into the study, including androgen deprivation therapy (ADT), androgen receptor pathway inhibition (ARPI) therapy, investigational agents, chemotherapy and other radioligand therapies such as alpha and beta-emitters (225Ac and 177Lu-based therapies, respectively). Most trial participants had received chemotherapy (67%, 10/15) and the median number of lines of therapy prior to receiving 67Cu-SAR-bisPSMA was 4. The median prostate-specific antigen (PSA) at study entry was 117.1 ng/ml (range 0.11-1,494.2).
The first 3 cohorts in the dose escalation phase of the trial were successfully completed with no DLTs reported in any of the participants dosed. No adverse events (AEs) related to 64Cu-SAR-bisPSMA were observed. Most AEs related to 67Cu-SAR-bisPSMA were low grade (grade 1 or 2). The most common AE reported was mild dry mouth (grade 1, 4/15 participants, 27%). The most common grade 3 AE was anaemia, reported in 2/15 participants (13%).
Preliminary data shows that despite having high levels of PSA and having received multiple treatments, 60% (9/15) of participants across all cohorts (including the lowest dose cohort of 67Cu-SAR-bisPSMA at 4GBq) showed reductions in PSA levels of greater than 35% from a single therapy cycle of 67Cu-SAR-bisPSMA. PSA reductions of greater than 80% were seen in 27% of all trial participants. In cohorts 2 and 3 (8 and 12GBq, respectively), PSA reductions of greater than 35% were observed in almost 80% (78%, 7/9) of participants and PSA was reduced by over 80% in 44% (4/9) of participants so far.
Clarity’s Executive Chairperson, Dr Alan Taylor, commented, “Treating the first participant in cohort 4 just a week after announcing the safety review from cohort 3 and opening cohort 4 is remarkable. With all available slots now allocated in the first part of cohort 4, we look forward to completing this final cohort in the dose escalation stage of the trial and moving to dose expansion, where we plan to enrol 14 patients. This high pace of recruitment into the SECuRE trial reflects the high unmet need in the prostate cancer therapy space as well as clinicians’ excitement about our SAR-bisPSMA product.
“Results from the first 3 cohorts and the data from 2 patients who received additional doses of 67Cu-SAR-bisPSMA under the US Expanded Access Program (EAP) are outstanding. These 2 patients from the EAP received 1 or 3 additional doses of 67Cu-SAR-bisPSMA at 8GBq or 4GBq, respectively2,3. They had failed multiple lines of therapy prior to being treated with 67Cu-SAR-bisPSMA and have had dramatic responses (94% reduction in PSA levels in one patient, and reduction to undetectable PSA levels in another) with only a few mild or moderate side effects from the treatment and excellent quality of life. This demonstrates great efficacy and a favourable safety profile of our product with the multi-dosing treatment. We look forward to replicating those results in cohort 4 and in the dose expansion phase of the trial at the higher dose of 12GBq. The data to date continues to reinforce our strong belief that we have a best-in-class radiopharmaceutical therapy, with dramatic responses obtained in patients that had failed multiple treatments, including other radiopharmaceutical therapies, as well as all other standard of care therapies and products in development. Coupled with the favourable safety profile, we believe we are well on our way to fulfilling our promise of improving treatment outcomes for people with cancer.”
Overview of Clarity’s SAR-bisPSMA clinical program
About SAR-bisPSMA
SAR-bisPSMA derives its name from the word “bis”, which reflects a novel approach of connecting two PSMA-targeting agents to Clarity’s proprietary sarcophagine (SAR) Technology that securely holds copper isotopes inside a cage-like structure, called a chelator. Unlike other commercially available chelators, the SAR Technology prevents copper leakage into the body. SAR-bisPSMA is a TCT that can be used with isotopes of copper-64 (Cu-64 or 64Cu) for imaging and copper-67 (Cu-67 or 67Cu) for therapy.
64Cu-SAR-bisPSMA and 67Cu-SAR-bisPSMA are unregistered products. The data outlined in this announcement has not been assessed by health authorities such as the US Food and Drug Administration (FDA). A clinical development program is currently underway to assess the efficacy and safety of these products. There is no guarantee that these products will become commercially available.
About Prostate Cancer
Prostate cancer is the second most common cancer diagnosed in men globally and the fifth leading cause of cancer death worldwide4. The American Cancer Institute estimates in 2024 there will be 299,310 new cases of prostate cancer in the US and around 35,250 deaths from the disease5.
About Clarity Pharmaceuticals
Clarity is a clinical stage radiopharmaceutical company focused on the treatment of serious disease. The Company is a leader in innovative radiopharmaceuticals, developing targeted copper theranostics based on its SAR Technology Platform for the treatment of cancer in children and adults.
References
- Clinicaltrials.gov Identifier: NCT04868604. clinicaltrials.gov/ct2/show/NCT04868604
- Clarity’s theranostic prostate cancer trial advances to highest dose level. Announcement. 10 Aug 2023. claritypharmaceuticals.com/news/secure_cohort3/
- First patient with metastatic prostate cancer to receive 2 doses of Cu-67 SAR-bisPSMA achieves undetectable PSA level. Announcement, 30 Nov 2023. claritypharmaceuticals.com/news/sar-bispsmaundetectablepsa/
- Global Cancer Statistics 2020. GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. acsjournals.onlinelibrary.wiley.com/doi/10.3322/caac.21660
- American Cancer Society Key Statistics for Prostate Cancer. cancer.org/cancer/prostate-cancer/about/key-statistics.html
Media Contacts
Clarity Pharmaceuticals
Dr Alan Taylor
Executive Chairperson
+61 (0)413 871 165
ataylor@claritypharm.com
Citadel-MAGNUS
Catherine Strong
Investor/Media Relations
+61 (0)406 759 268
cstrong@citadelmagnus.com
This announcement has been authorised for release by the Executive Chairperson.
