Sydney, Australia 30 November 2023
Highlights
- Undetectable levels of Prostate Specific Antigen (PSA) have been reported from the first patient with metastatic castrate-resistant prostate cancer (mCRPC) to ever receive two cycles of Clarity’s 67Cu-SAR-bisPSMA at the 8GBq dose level. PSA is a marker of tumour burden, clinical response to treatment and an indicator of the recurrence of disease for prostate cancer.
- The patient also had undetectable lesions using PET post-treatment, with two lesions showing a complete response (absence of all detectable cancer), and one lesion showing a partial response, missing the complete response criteria by 2 millimetres, based on Response Evaluation Criteria In Solid Tumours (RECIST) assessment.
- The patient was a participant in cohort 2 of Clarity’s ongoing SECuRE trial evaluating 64Cu-SAR-bisPSMA and 67Cu-SAR-bisPSMA in mCRPC. The participant was also heavily pre-treated with multiple lines of therapy, including androgen deprivation therapy, ARPI, chemotherapy and a PARP inhibitor and had failed all previous treatments.
- The patient received the two cycles of 8GBq 67Cu-SAR-bisPSMA with the initial cycle provided under the SECuRE trial protocol and the second cycle provided under the US Food and Drug Administration’s (FDA) Expanded Access Program (EAP). The patient experienced three adverse events, most mildly, and all have resolved.
- No dose limiting toxicities (DLTs) have been reported in any of the 12 patients treated in the SECuRE trial to date and recruitment is ongoing for cohort 3 at the highest single dose level of 12GBq.
Clarity Pharmaceuticals (ASX: CU6) (“Clarity”, “the Company”), a clinical stage radiopharmaceutical company with a mission to develop next-generation products that improve treatment outcomes for children and adults with cancer, is pleased to announce that the first patient ever to be dosed with two cycles of 8GBq of 67Cu-SAR-bisPSMA has had a drop in PSA to undetectable levels, undetectable disease using PET and a near complete response to treatment under RECIST. The patient received the first cycle as part of cohort 2 of Clarity’s theranostic trial, SECuRE, evaluating 64Cu/67Cu-SAR-bisPSMA in patients with mCRPC, and a second cycle under the US FDA EAP, as requested by the patient’s clinician. The patient experienced a mild dry mouth, altered taste and moderate fatigue, all adverse events resolved themselves.
Following the administration of two cycles of 67Cu-SAR-bisPSMA at the 8GBq dose level, the near complete response (absence of all detectable cancer after treatment) was reported following the Response Evaluation Criteria In Solid Tumours (RECIST) assessment – Figures 1 and 2. The patient had already failed multiple lines of treatment, including hormone therapy, an investigational agent and chemotherapy prior to being treated with 67Cu-SAR-bisPSMA.
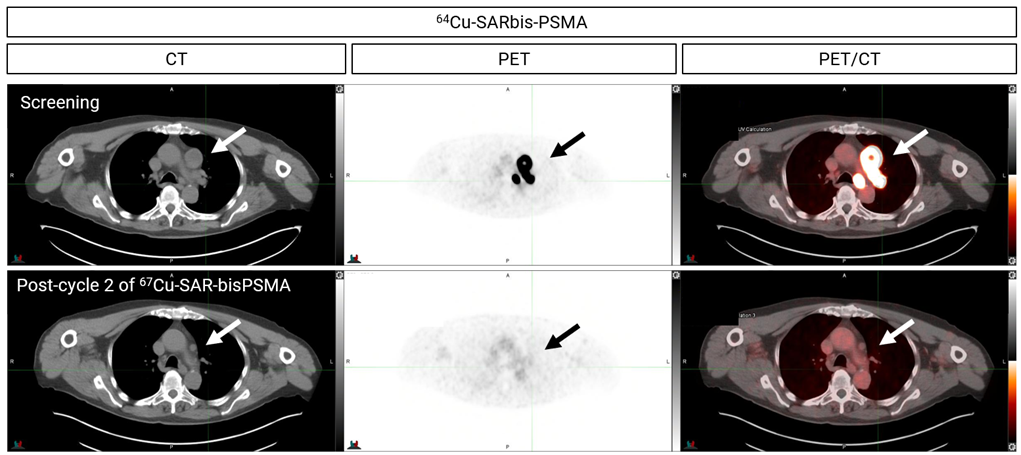
Figure 1. PET/CT images showing uptake of 64Cu-SAR-bisPSMA at screening in a patient with mCRPC (top). The patient received 2 cycles of 67Cu-SAR-bisPSMA at 8 GBq. Images post-treatment show no 64Cu-SAR-bisPSMA uptake (bottom).
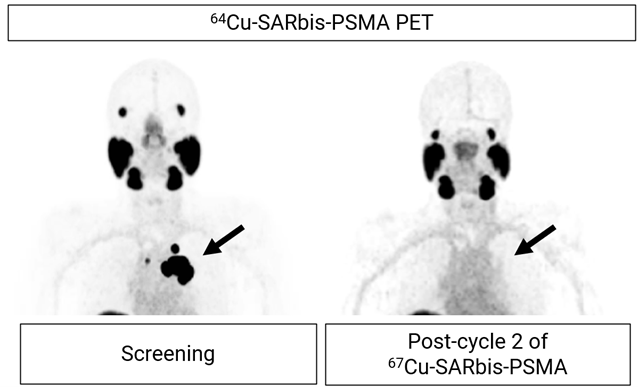
Figure 2. PET images showing uptake of 64Cu-SAR-bisPSMA at screening in a patient with mCRPC (left; SUVmax 140.1. SUV: standardised uptake value). The patient received 2 cycles of 67Cu-SAR-bisPSMA at 8 GBq. Images post-treatment show no 64Cu-SAR-bisPSMA uptake (right).
The patient had a reduction in PSA levels from 47.2 ng/L at baseline to an undetectable level of less than 0.05 ng/ml – Figure 3. PSA is a well characterised marker of tumour burden, clinical response to treatment and an indicator of the recurrence of disease for prostate cancer. Moreover, PSA decline is an independent prognostic indicator of improved overall survival following radioligand therapy2,3.
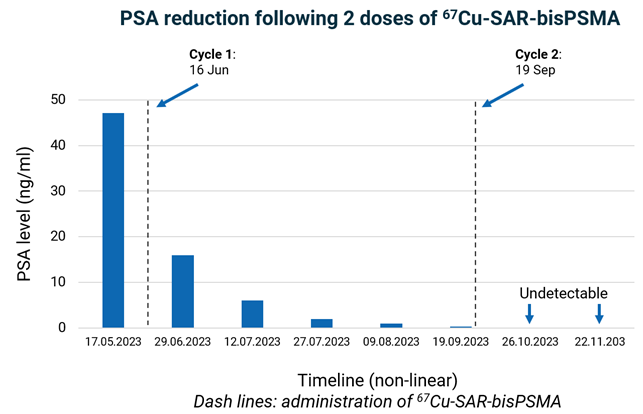
Figure 3. PSA dynamics over time. Series of PSA test results show baseline and decrease over time after the administration of one cycle of 67Cu-SAR-bisPSMA. PSA level was undetectable in the last 2 measurements after the second cycle of 67Cu-SAR-bisPSMA. Lower level of detection: 0.05 ng/ml.
As previously reported, one third of participants (2/6) in the SECuRE trial cohort 1, the lowest dose cohort of 4GBq, had a PSA decline greater than 50% from a single cycle of 67Cu-SAR-bisPSMA. In cohort 2, all participants (3/3) had a PSA decline greater than 80% from a single 8GBq cycle, with two showing greater than 95% drops in PSA. In cohort 3, two thirds of participants (2/3) showed reductions in PSA levels of approximately 40% and over 90% each. One of the patients in cohort 3 will be soon receiving his second dose of 67Cu-SAR-bisPSMA at 12GBq under EAP. A PSA drop of 50% or greater is one of the primary endpoints of the SECuRE trial and a commonly used surrogate endpoint for efficacy in this patient population. Importantly, no DLTs have been reported in the SECuRE trial in any of the patients dosed with 67Cu-SAR-bisPSMA to date. Recruitment is ongoing into cohort 3 of the trial where the dose level has been increased to the highest single dose level of 12GBq.
Dr Luke Nordquist, CEO, Urologic Medical Oncologist and Principal Investigator at the Urology Cancer Center / XCancer Omaha, NE, commented, “As a clinician, there is nothing more rewarding than delivering the news to your patient that their cancer can no longer be detected, and with very few side effects following treatment, particularly for a patient that was heavily pre-treated with multiple lines of therapy, including androgen deprivation therapy, ARPI, chemotherapy and a PARP inhibitor and had failed all these treatments. For this patient who received two cycles of 67Cu-SAR-bisPSMA at 8GBq, we have confirmed a near complete response through RECIST and have seen the result reflected in the reduction of PSA to an undetectable level and undetectable disease using PET. The EAP is giving us insights into the potential therapeutic benefit we might see from multiple doses of 67Cu-SAR-bisPSMA, and the early data is showing that 67Cu-SAR-bisPSMA may one day provide cancer patients with an effective and safe alternative for those in need. I believe 67Cu-SAR-bisPSMA presents a new opportunity for cancer patients to have an effective result with few side effects.”
Clarity’s Executive Chairperson, Dr Alan Taylor, commented, “We are very pleased to have already observed undetectable PSA levels, undetectable disease using PET and only failing a complete response based on RECIST by 2 millimetres in one lesion. This result is following the patient’s failure to respond to multiple lines of therapy, including hormone therapy, an investigational agent and chemotherapy, with very few side effects that were mostly mild and reversed. This is a very exciting result for the very first time we have dosed a patient twice at what we would consider a therapeutic dose level of 8GBq. Although we are still progressing through the dose-escalation phase of the trial, the near complete response following RECIST and undetectable PSA are very encouraging. The favourable safety profile of the product, in a patient with this level of response, is a very welcome reward for the hard work and dedication of our team and collaborators that has led to this early success.
“But we are still in the midst of development for this product, and although it has been a very exciting start, we will continue to focus on the rapid completion of this trial as we move towards achieving our ultimate goal of better treating many patients with this insidious disease.”
About the SECuRE Trial
The SECuRE trial (NCT04868604)1 is a Phase I/IIa theranostic trial for identification and treatment of Prostate-Specific Membrane Antigen (PSMA) expressing mCRPC using Targeted Copper Theranostics (TCTs). 64Cu-SAR-bisPSMA is used to visualise PSMA expressing lesions and select candidates for subsequent 67Cu-SAR-bisPSMA therapy. The trial is a multi-centre, single arm, dose escalation trial with a cohort expansion involving up to 44 patients in the US. The aim of this trial is to determine the safety and efficacy of 67Cu SAR-bisPSMA for the treatment of prostate cancer.
About SAR-bisPSMA
SAR-bisPSMA derives its name from the word “bis”, which reflects a novel approach of connecting two PSMA binding motifs to Clarity’s proprietary sarcophagene (SAR) technology that securely holds copper isotopes inside a cage-like structure, called a chelator. Unlike other commercially available chelators, the SAR technology prevents copper leakage into the body. SAR-bisPSMA is a TCT that can be used with isotopes of copper-64 (Cu-64 or 64Cu) for imaging and copper-67 (Cu-67 or 67Cu) for therapy.
64Cu-SAR-bisPSMA and 67Cu-SAR-bisPSMA are unregistered products. Individual results may not represent the overall safety and efficacy of the products. The data outlined in this announcement has not been assessed by health authorities such as the FDA. A clinical development program is currently underway to assess the efficacy and safety of these products. There is no guarantee that these products will become commercially available.
Overview of Clarity’s SAR-bisPSMA clinical program
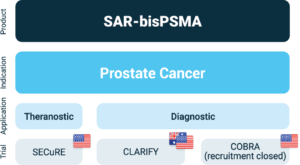
About Prostate Cancer
Prostate cancer is the second most common cancer diagnosed in men globally and the fifth leading cause of cancer death worldwide4. The American Cancer Institute estimates in 2023 there will be 288,300 new cases of prostate cancer in the US and around 34,700 deaths from the disease5.
About Clarity Pharmaceuticals
Clarity is a clinical stage radiopharmaceutical company focused on the treatment of serious disease. The Company is a leader in innovative radiopharmaceuticals, developing TCTs based on its SAR technology platform for the treatment of children and adults with cancer.
References
- ClinicalTrials.gov Identifier: NCT04868604 https://clinicaltrials.gov/ct2/show/NCT04868604
- Rahbar K et al. PSMA targeted radioligand therapy in metastatic castration resistant prostate cancer after chemotherapy, abiraterone and/or enzalutamide. A retrospective analysis of overall survival. Eur J Nucl Med Mol Imaging, 2018.
- Ahmadzadehfar H et al. Overall survival and response pattern of castration-resistant metastatic prostate cancer to multiple cycles of radioligand therapy using [177Lu]Lu-PSMA-617. Eur J Nucl Med Mol Imaging, 2017.
- Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries, https://acsjournals.onlinelibrary.wiley.com/doi/10.3322/caac.21660
- American Cancer Society: Key Statistics for Prostate Cancer, https://www.cancer.org/cancer/prostate-cancer/about/key-statistics.html
Media Contacts
Clarity Pharmaceuticals
Dr Alan Taylor
Executive Chairperson
+61 (0)413 871 165
ataylor@claritypharm.com
Citadel-MAGNUS
Catherine Strong
Investor/Media Relations
+61 (0)406 759 268
cstrong@citadelmagnus.com
This announcement has been authorised for release by the Executive Chairperson.
