Sydney, Australia 12 September 2022
Clarity Pharmaceuticals (ASX: CU6) (“Clarity”), a clinical-stage radiopharmaceutical company with a mission to develop next-generation products that improve treatment outcomes for children and adults with cancer, is pleased to announce that the patent application covering Clarity’s optimised Prostate Specific Membrane Antigen (PSMA) targeting agent, SAR-bisPSMA, has been Granted in China.
The patent had been previously granted in the USA, Australia and Mexico and has an expiry date of 5 June 2038. The patent application remains under review in other major jurisdictions, including Europe and Japan.
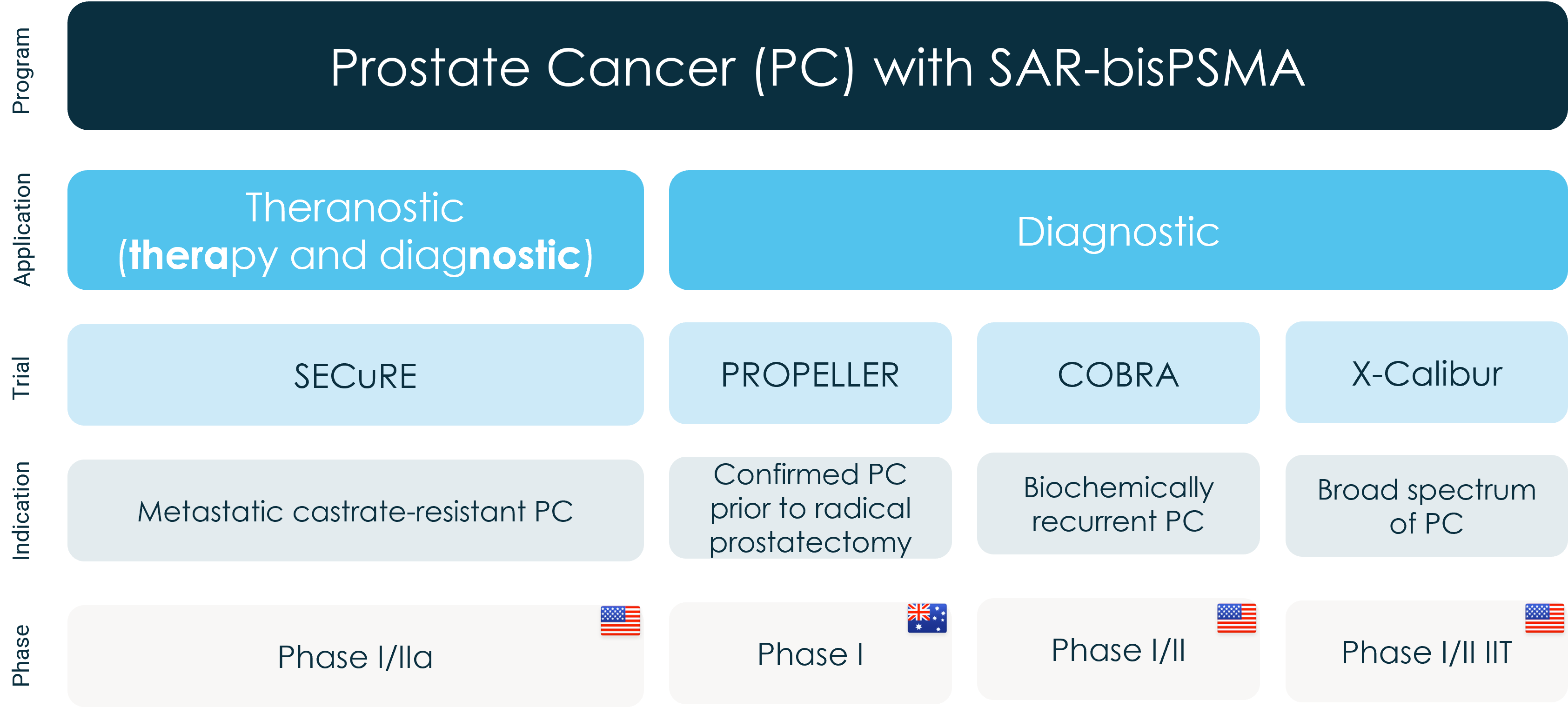
SAR-bisPSMA is a Targeted Copper Theranostic (TCT) for diagnosis and treatment of prostate cancer as well as other cancers expressing PSMA. The prostate cancer market is a key focus for Clarity as the company continues to hit key milestones in the development of SAR-bisPSMA in three clinical trials:
SECuRE theranostic trial in the US
- Phase I/IIa theranostic study of 64Cu-SAR-bisPSMA and 67Cu-SAR-bisPSMA for identification and treatment of PSMA-expressing metastatic castrate resistant prostate cancer (mCRPC) (NCT04868604)1
- Status: Therapy phase open for recruitment
PROPELLER diagnostic trial in Australia
- Positron Emission Tomography (PET) imaging of participants with confirmed prostate cancer using 64Cu-SAR-bisPSMA: a multi-centre, blinded review, dose ranging Phase I study (NCT04839367)2
- Status: Recruitment complete
COBRA diagnostic trial in the US
- Phase I/II PET imaging trial of participants with biochemical recurrence (BCR) of prostate cancer following definitive therapy using 64Cu SAR-bisPSMA in the US (NCT05249127)3
- Status: Recruiting
Clarity’s Executive Chairman, Dr Alan Taylor, commented, “We are very pleased with our broad and evolving patent protection for all of our products, but in particular for one of our core products, with this composition-of-matter patent now granted in the USA, Australia, China and Mexico. This further strengthens our position as we progress the clinical development of SAR-bisPSMA. This milestone continues Clarity’s strong emphasis on Intellectual Property (IP) protection covering the SAR Technology platform and each new product that stems from it.
“Clarity’s aggressive patenting strategy enables us to successfully leverage our IP to achieve strong protection and develop a pipeline of next-generation radiopharmaceuticals. This allows us to gain a sustainable competitive advantage in the field as we continue pursuing our ultimate goal of developing better treatments for children and adults with cancer.”
Clarity’s SAR-bisPSMA clinical trial program overview
About SAR-bisPSMA
SAR-bisPSMA derives its name from the word “bis”, which reflects a novel approach of connecting two prostate-specific membrane antigen (PSMA) binding motifs to Clarity’s proprietary sarcophagene (SAR) technology that securely holds copper isotopes inside a cage-like structure, called a chelator. Unlike other commercially available chelators, the SAR technology prevents copper leakage into the body. SAR-bisPSMA is a Targeted Copper Theranostic (TCT) that can be used with isotopes of copper-64 (Cu-64 or 64Cu) for imaging and copper-67 (Cu-67 or 67Cu) for therapy.
About Prostate Cancer
Prostate cancer is the second most common cancer diagnosed in men globally and the fifth leading cause of cancer death worldwide4. The National Cancer Institute estimates in 2022 there will be 268,490 new cases of prostate cancer in the US and around 34,500 deaths from the disease5.
About Clarity
Clarity is a clinical stage radiopharmaceutical company focused on the treatment of serious disease. The Company is a leader in innovative radiopharmaceuticals, developing targeted copper theranostics based on its SAR Technology Platform for the treatment of cancer in children and adults.
www.claritypharmaceuticals.com
References
- Clinicaltrials.gov Identifier: NCT04868604 https://clinicaltrials.gov/ct2/show/NCT04868604
- Clinicaltrials.gov Identifier: NCT04839367 https://clinicaltrials.gov/ct2/show/NCT04839367
- Clinicaltrials.gov Identifier: NCT05249127 https://clinicaltrials.gov/ct2/show/NCT05249127
- Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries https://acsjournals.onlinelibrary.wiley.com/doi/10.3322/caac.21660
- American Cancer Society, Cancer Statistics Center, https://cancerstatisticscenter.cancer.org/?_ga=2.79808020.284532473.1620009137-1916069442.1615761164#!/cancer-site/Prostate
Media Contacts
Clarity Pharmaceuticals
Dr Alan Taylor
Executive Chairman
+61 (0)413 871 165
ataylor@claritypharm.com
Citadel-MAGNUS
Catherine Strong
Investor/Media Relations
+61 (0)406 759 268
cstrong@citadelmagnus.com
This announcement has been authorised for release by the Executive Chairman.
