Sydney, Australia 14 December 2022
Highlights
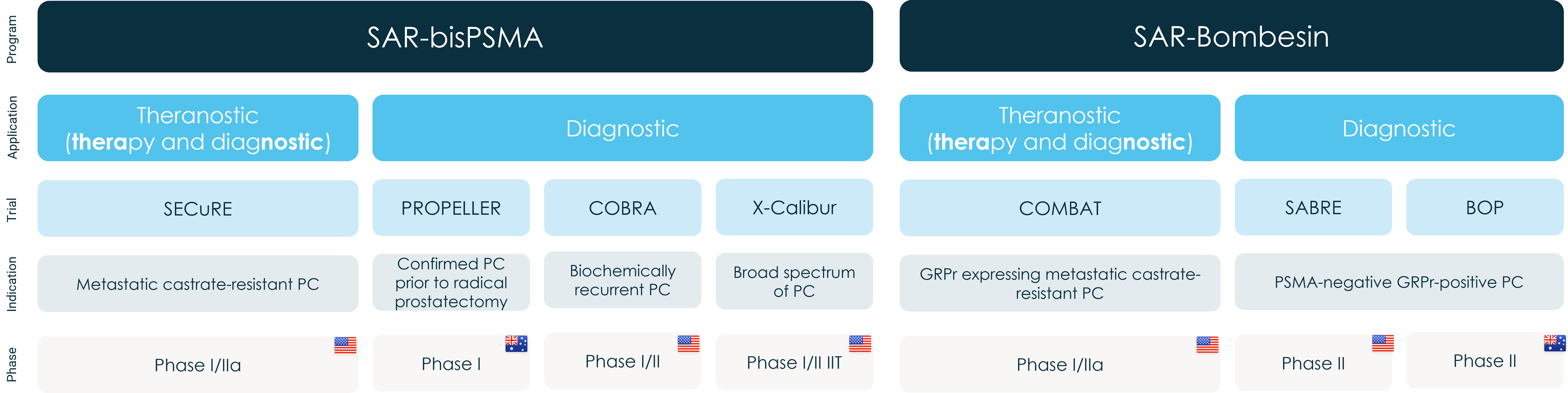
- Clarity’s diagnostic 64Cu SAR-bisPSMA Phase I trial PROPELLER meets all primary and secondary objectives
- 64Cu SAR-bisPSMA is safe and well tolerated in trial participants
- 64Cu SAR-bisPSMA is shown to be efficacious in the detection of primary prostate cancer
- A dose of 200 megabecquerels (MBq) of 64Cu SAR-bisPSMA was determined as the optimal dose for future trials
- Initial data on 64Cu SAR-bisPSMA will be released at the American Society of Clinical Oncology (ASCO) Genitourinary (GU) Symposium in February 2023
- Data from the PROPELLER trial is being used to plan the definitive Phase III clinical trial scheduled to commence in 2023
Clarity Pharmaceuticals (ASX: CU6) (“Clarity”), a clinical-stage radiopharmaceutical company with a mission to develop next-generation products that improve treatment outcomes for children and adults with cancer, announces positive topline results from its diagnostic Phase I trial of 64Cu SAR-bisPSMA in prostate cancer (PROPELLER NCT048393671).
The trial met its primary objectives and the 64Cu SAR-bisPSMA product was found to be safe, well tolerated and efficacious in detecting primary prostate cancer. PROPELLER also met its secondary objective of determining the optimal dose for subsequent investigation of 64Cu SAR-bisPSMA. The selected optimal dose level of 200 MBq is currently applied in all ongoing trials.
The PROPELLER trial was a first-in-human Positron Emission Tomography (PET) imaging trial of participants with confirmed prostate cancer using Clarity’s optimised PSMA agent, 64Cu SAR-bisPSMA. It was designed as a multi-centre, blinded review, dose ranging, non-randomised study administered to 30 participants with confirmed prostate cancer prior to undergoing radical prostatectomy. The trial also compared the diagnostic properties of 64Cu SAR-bisPSMA against 68Ga PSMA-11, which is approved for prostate cancer imaging in Australia and the US.
Primary objectives
- Safety and tolerability of 64Cu-SAR-bisPSMA using the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0.
- Efficacy of 64Cu-SAR-bisPSMA in the detection of primary prostate cancer compared to histopathology.
Secondary objectives
- Assessment of image quality at varying dose levels of 64Cu SAR-bisPSMA for (100 MBq, 150 MBq and 200 MBq).
Clarity’s Executive Chairman, Dr Alan Taylor, commented, “The initial PROPELLER data further substantiates the utility of 64Cu SAR-bisPSMA in the diagnosis of prostate cancer. Combined with our clinical and pre-clinical trial data to date, this validates SAR-bisPSMA as a potential best-in-class PSMA agent for the diagnosis (with 64Cu) and subsequent treatment (with 67Cu) of prostate cancer. As such, we are thrilled to continue the development of SAR-bisPSMA as a theranostic pair in our SECuRE trial2 as well as in two diagnostic prostate cancer indications: pre-prostatectomy/pre-definitive treatment and suspected biochemical recurrence of the disease (COBRA trial3). We have already commenced work towards our diagnostic Phase III trials with 64Cu SAR-bisPSMA and we look forward to engaging with the United States Food and Drug Administration shortly as we get closer to our ultimate goal of improving treatment outcomes of people with cancer.”
Prof Louise Emmett, (St Vincent’s Hospital Sydney), Principal Investigator in the PROPELLER trial, commented, “We are impressed by the high uptake of 64Cu SAR-bisPSMA and look forward to progressing its development and further analysing the data. We have seen to date the high uptake of the product in tumours and the ability to image 64Cu SAR-bisPSMA at later time points, which may improve the treatment paradigm for many men with prostate cancer. Being able to accurately stage cancer means that we can better develop a treatment approach that can more effectively prevent its further spread throughout the body. We are excited to continue analysing the data and present the initial results at the ASCO GU Symposium in February 2023.”
Clarity’s Prostate Cancer clinical trial program overview
About SAR-bisPSMA
SAR-bisPSMA derives its name from the word “bis”, which reflects a novel approach of connecting two prostate-specific membrane antigen (PSMA) binding motifs to Clarity’s proprietary sarcophagene (SAR) technology that securely holds copper isotopes inside a cage-like structure, called a chelator. Unlike other commercially available chelators, the SAR technology prevents copper leakage into the body. SAR-bisPSMA is a Targeted Copper Theranostic (TCT) that can be used with isotopes of copper-64 (Cu-64 or 64Cu) for imaging and copper-67 (Cu-67 or 67Cu) for therapy.
About Prostate Cancer
Prostate cancer is the second most common cancer diagnosed in men globally and the fifth leading cause of cancer death worldwide4. The National Cancer Institute estimates in 2022 there will be 268,490 new cases of prostate cancer in the US and around 34,500 deaths from the disease5.
About Clarity
Clarity is a clinical stage radiopharmaceutical company focused on the treatment of serious disease. The Company is a leader in innovative radiopharmaceuticals, developing targeted copper theranostics based on its SAR Technology Platform for the treatment of cancer in children and adults.
www.claritypharmaceuticals.com
References
- ClinicalTrials.gov Identifier: NCT04839367 https://clinicaltrials.gov/ct2/show/NCT04839367
- ClinicalTrials.gov Identifier: NCT04868604 https://clinicaltrials.gov/ct2/show/NCT04868604
- ClinicalTrials.gov Identifier: NCT05249127 https://clinicaltrials.gov/ct2/show/NCT05249127
- Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries https://acsjournals.onlinelibrary.wiley.com/doi/10.3322/caac.21660
- American Cancer Society, Cancer Statistics Center, https://cancerstatisticscenter.cancer.org/?_ga=2.79808020.284532473.1620009137-1916069442.1615761164#!/cancer-site/Prostate
Media Contact
Clarity Pharmaceuticals
Dr Alan Taylor
Executive Chairman
+61 (0)413 871 165
ataylor@claritypharm.com
Citadel-MAGNUS
Catherine Strong
Investor/Media Relations
+61 (0)406 759 268
cstrong@citadelmagnus.com
This announcement has been authorised for release by the Executive Chairman.
