Sydney, Australia 7 August 2023
Clarity Pharmaceuticals (ASX: CU6) (“Clarity”, “the Company”), a clinical stage radiopharmaceutical company with a mission to develop next-generation products that improve treatment outcomes for children and adults with cancer, is pleased to announce an exclusive license from Memorial Sloan Kettering Cancer Center (MSK). The license is to intellectual property that covers cutting-edge technology that enables antibody “pre-targeting” for the diagnosis and treatment of cancer.
Pre-targeting is a radiopharmaceutical approach to diagnosing and treating cancer patients that harnesses the benefits of antibody targeting, amplifying uptake of radiopharmaceutical products in cancerous tissue, while reducing healthy tissue exposure to radiation that can arise due to the slow clearance of antibodies. This is achieved by tagging an antibody, designed specifically to target cancer cells, and then injecting it into the body. After several days, a chaser compound, which only attaches to the antibody tag, is injected. The chaser compound is initially radiolabelled with copper-64 to enable imaging with a Positron Emission Tomography (PET) camera which visualises the extent of cancer burden. Once the cancer is visualised, a second administration of the chaser is administered, this time radiolabelled with the therapeutic radionuclide copper-67, so that the cancer cells can be irradiated with the goal of killing the tumours.
The ability of the chaser compound to attach to the tag on the antibody occurs due to a “click chemistry” reaction. This chemistry is so groundbreaking, it was awarded a Nobel Prize in Chemistry to scientists Carolyn R. Bertozzi, Morten Meldal and K. Barry Sharpless in 2022 for Click Chemistry and Bioorthogonal Chemistry.
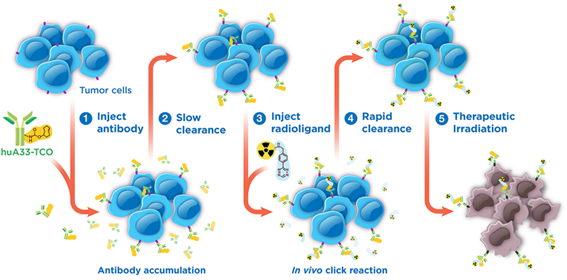
Figure 1. This pre-targeting therapy starts with an antibody ‘tagged’ with TCO. The antibody tagged-TCO is injected in the body and binds to cancer cells (1). The unbound antibody slowly clears the body (2) so that there is primarily binding to the cancer cells with limited background. After a few days, the radioligand (the chaser compound) is injected (3) in the body and via the “click” reaction, attaches to the TCO tag on the antibody. Unbound radioligand otherwise clears the body quickly (4). The bound antibodies, now radiolabeled, irradiate the cancer cells with a therapeutic dose (5).
This technology, developed at MSK by Jason Lewis, PhD and Dr Brian Zeglis (formerly MSK, currently Hunter College, NY USA), has been licensed to Clarity under a worldwide exclusive license. Key publications on the pre-targeting research include PNAS1 and NEJM2. This IP complements a growing number of patent applications in Clarity’s portfolio covering various aspects of antibody pre-targeting: International Application No.PCT/AU2019/050322 (assigned to Clarity from the University of Melbourne); International Application No.PCT/AU2019/050324; and Australian Provisional Patent Application No. 2022903384.
A clinical trial using the MSK licensed technology is open for recruitment in patients with pancreatic cancer at MSK headed by Dr Pandit-Taskar (NCT05737615)3. The trial is titled: “PET Imaging Using 64Cu-Tz-SarAr and hu5B1-TCO in People With Pancreatic Cancer”. It is a first-in-human diagnostic trial, utilising copper-64 and a sarcophagine chelator, core to Clarity’s SAR Technology. The antibody (hu5B1-TCO) being used targets pancreatic cancer.
Clarity’s Executive Chairman, Dr Alan Taylor, commented, “Pre-targeting presents an exciting and groundbreaking avenue to pursue our mission to develop next-generation products that improve treatment outcomes for children and adults with cancer. Pre-targeting holds promise of overcoming the safety issues of antibody-based radiopharmaceuticals, opening up a massive opportunity to use the large cache of antibodies developed over the last 20 years and applying it in the theranostic setting. Combining this approach with the “perfect pairing” of copper-64 and copper-67 represents an untapped opportunity to deliver significant payloads to cancers in a safer and more effective way as we start to turn the tide on our war with cancer.
“Pre-targeting using copper-64 in MSK’s first-in-human trial is in alignment with Clarity’s clinical and commercial development of the SAR Technology to make a pipeline of theranostic products. The trial will validate the technique, and, if positive, will pave the way for a whole new area of development, not only for Clarity, but the entire field of antibody-based therapy. We have valued our engagement with MSK on many fronts over the years and we look forward to progressing the development of MSK’s pre-targeting technology, potentially providing a new path for the accurate and precise detection and treatment of various cancers.”
Dr Jason Lewis, Emily Tow Chair in Oncology and Vice Chair for Research, Department of Radiology at MSK, commented, “We have been conducting research on pre-targeting for many years now and are very excited with the impending first-in-human clinical trial by our team here at MSK. Pre-targeting is a promising method of treatment for cancer patients and the continued exploration and validation of the benefits associated with it in a clinical setting is the next stage for this field. To have Clarity involved now on the clinical and commercial development of a product is excellent and is a continuation of their support and input on the preclinical research to date. Although pre-targeting has been in humans in the past, using various techniques, the field is still in its infancy. The theranostic approach in pre-targeting with the next generation of isotopes, such as copper-64 and copper-67, provides a renewed interest in the field, with a focus on both diagnosis and therapy. This technique has the ability to widen the whole field of nuclear medicine and utilise a large array of existing knowledge on antibody therapies.”
Dr Lewis has financial interests related to Clarity Pharmaceuticals.
About Clarity Pharmaceuticals
Clarity is a clinical stage radiopharmaceutical company focused on the treatment of serious disease. The Company is a leader in innovative radiopharmaceuticals, developing TCTs based on its SAR technology platform for the treatment of children and adults with cancer.
References
- Keinänen O, Fung K, Brennan JM, Zia N, Harris M, van Dam E, Biggin C, Hedt A, Stoner J, Donnelly PS, Lewis JS, Zeglis BM. Harnessing 64Cu/67Cu for a theranostic approach to pretargeted radioimmunotherapy. Proc Natl Acad Sci U S A. 2020 Nov 10;117(45):28316-28327.
- Zeglis BM, Lewis JS. Click Here for Better Chemistry. N Engl J Med. 2022 Dec 15;387(24):2291-2293.
- ClinicalTrials.gov Identifier: NCT05737615. https://clinicaltrials.gov/ct2/show/NCT05737615
Media Contacts
Clarity Pharmaceuticals
Dr Alan Taylor
Executive Chairman
+61 (0)413 871 165
ataylor@claritypharm.com
Citadel-MAGNUS
Catherine Strong
Investor/Media Relations
+61 (0)406 759 268
cstrong@citadelmagnus.com
This announcement has been authorised for release by the Executive Chairman.
