Sydney, Australia 14 June 2023
Clarity Pharmaceuticals (ASX: CU6) (“Clarity”, “the Company”), a clinical stage radiopharmaceutical company with a mission to develop next-generation products that improve treatment outcomes for children and adults with cancer, and PETNET Solutions Inc, a Siemens Healthineers Company – a global positron emission tomography (PET) radiopharmaceutical network and the leading manufacturer of radiopharmaceuticals for PET imaging in the US – have entered into a Master Service Agreement and a Clinical Supply Agreement covering Clarity’s 64Cu SAR-bisPSMA, a next-generation diagnostic radiopharmaceutical product for prostate cancer imaging.
Under the Clinical Supply Agreement, PETNET Solutions will manufacture ready-to-use 64Cu SAR-bisPSMA according to the current Good Manufacturing Practice (cGMP) guidelines for Clarity’s US-based clinical trials. 64Cu SAR-bisPSMA is an investigational PET agent anticipated to be entering a pivotal Phase III trial in the US before the end of 2023 and, potentially, a second Phase III trial in 2024. The Master Services Agreement between the companies will further enable a streamlined technology transfer of other Cu-64 based imaging agents from Clarity to the PETNET Solutions network in the US for current and future clinical trials.
Clarity’s Executive Chairman, Dr Alan Taylor, commented, “We are excited to be working with the largest PET manufacturing network in the US with regards to our 64Cu SAR-bisPSMA product. The prostate cancer market is one of the largest in oncology, and the growing US market for PSMA PET diagnostics is anticipated to generate sales of US$1.0 Billion during 2023.
“We spent time and effort optimising our proprietary PSMA agent and our trials to date with 64Cu SAR-bisPSMA have already shown increased uptake of the product into lesions compared to first-generation PSMA agents, as well as more robust retention of the product over time. These advantages facilitate clinically relevant later time point imaging (>24 hours), a feature not possible with the current PSMA agents that utilise short half-life isotopes of gallium and fluorine. These three unique characteristics of increased uptake, robust retention and a clinically relevant half-life, work together to enhance lesion detection in the hope of overcoming the low sensitivity of first-generation PSMA agents. We are looking forward to commencing our registrational Phase III trials with PETNET Solutions and elucidating the many benefits of 64Cu SAR-bisPSMA to bring this next generation PSMA-PET diagnostic to prostate cancer patients around the world.”
Barry Scott, CEO of PETNET Solutions, commented, “PETNET Solutions is excited to work with Clarity to supply 64Cu SAR-bisPSMA for their Phase III clinical trials. With the right experience and network to help ensure that the Phase III clinical trials run seamlessly, we look forward to bringing 64Cu SAR-bisPSMA to prostate cancer patients, with the shared goal of improving treatment outcomes.”
PETNET Solutions will provide a dependable and scalable supply of 64Cu SAR-bisPSMA, allowing two stand-alone diagnostic Phase III clinical trials to proceed at a large number of clinical sites across the US. The longer 48-hour shelf-life of 64Cu SAR-bisPSMA enables centralised manufacture and supply for both planned Phase III trials from just one facility, as opposed to the first-generation PSMA PET diagnostics that require an expensive and extensive network of cyclotrons, radioisotope generators and radiopharmacies due to the shorter half-life of gallium-68 and fluorine-18.
The Master Service Agreement and the Clinical Supply Agreement are effective 13 June 2023. The initial supply from PETNET Solutions is expected to occur before the end of calendar year 2023. The Master Services Agreement is for an initial period of 5 years and the Clinical Supply Agreement is for an initial period of 3 years. Both may be extended upon mutual agreement of the parties. Cancellation provisions are at industry standard rates.
Clarity’s SAR-bisPSMA clinical trial program overview
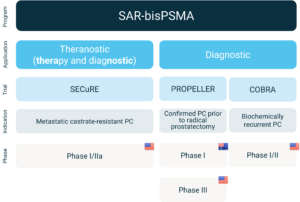
About SAR-bisPSMA
SAR-bisPSMA derives its name from the word “bis”, which reflects a novel approach of connecting two prostate-specific membrane antigen (PSMA) binding motifs to Clarity’s proprietary sarcophagene (SAR) technology that securely holds copper isotopes inside a cage-like structure, called a chelator. Unlike other commercially available chelators, the SAR technology prevents copper leakage into the body. SAR-bisPSMA is a Targeted Copper Theranostic (TCT) that can be used with isotopes of copper-64 (Cu-64 or 64Cu) for imaging and copper-67 (Cu-67 or 67Cu) for therapy.
About Prostate Cancer
Prostate cancer is the second most common cancer diagnosed in men globally and the fifth leading cause of cancer death worldwide1. The American Cancer Institute estimates in 2023 there will be 288,300 new cases of prostate cancer in the US and around 34,700 deaths from the disease2.
About Clarity Pharmaceuticals
Clarity is a clinical stage radiopharmaceutical company focused on the treatment of serious disease. The Company is a leader in innovative radiopharmaceuticals, developing targeted copper theranostics based on its SAR Technology Platform for the treatment of cancer in children and adults.
www.claritypharmaceuticals.com
References
- Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries, https://acsjournals.onlinelibrary.wiley.com/doi/10.3322/caac.21660
- American Cancer Society: Key Statistics for Prostate Cancer, https://www.cancer.org/cancer/prostate-cancer/about/key-statistics.html
Media Contact
Clarity Pharmaceuticals
Dr Alan Taylor
Executive Chairman
+61 (0)413 871 165
ataylor@claritypharm.com
Citadel-MAGNUS
Catherine Strong
Investor/Media Relations
+61 (0)406 759 268
cstrong@citadelmagnus.com
This announcement has been authorised for release by the Executive Chairman.
