Sydney, Australia 19 February 2025
Clarity Pharmaceuticals (ASX: CU6) (“Clarity” or “Company”), a clinical-stage radiopharmaceutical company with a mission to develop next-generation products that improve treatment outcomes for children and adults with cancer, is pleased to announce that the United States (US) Food and Drug Administration (FDA) has granted Fast Track Designation (FTD) for 67Cu-SAR-bisPSMA for the treatment of adult patients with prostate-specific membrane antigen (PSMA)-positive metastatic castration-resistant prostate cancer (mCRPC) who have been previously treated with androgen receptor pathway inhibition (ARPI).
This milestone builds on Clarity’s earlier receipt of 2 FTDs for the diagnostic 64Cu-SAR-bisPSMA in patients with suspected metastasis of prostate cancer who are candidates for initial definitive therapy1, as well as patients with biochemical recurrence (BCR) of prostate cancer following definitive therapy2, with 2 Phase III registration trials underway (CLARIFY [NCT06056830]3 and AMPLIFY, respectively). These 3 FTDs demonstrate the quality of the data generated to date on the 64Cu-SAR-bisPSMA and 67Cu-SAR-bisPSMA products in addressing serious unmet needs in prostate cancer. The FTDs will enable Clarity to accelerate the development of its comprehensive program with the optimised SAR-bisPSMA agent to be used in patients with prostate cancer throughout the management of their cancer, from initial to late-stage disease, with an opportunity to completely change the entire treatment landscape for the large prostate cancer market.
The FDA’s FTD is designed to expedite the development and regulatory review of novel drugs addressing serious conditions with significant unmet medical needs. For SAR-bisPSMA, it provides a number of product development advantages. The designations pave the way for a faster review process once Clarity submits its product approval applications. Additionally, it enables more frequent communication with the FDA, allowing for rapid resolution of queries during development. Furthermore, Clarity can submit completed sections of its application as they are ready, rather than waiting for the entire package to be finished before it can be lodged with the FDA. These benefits would reduce the review time needed to bring this innovative and proprietary molecule to the prostate cancer imaging and therapy markets.
The data for this FTD submission was based on the preliminary results to date from the Phase I/IIa SECuRE study (NCT04868604)4, which is investigating the safety and efficacy of 67Cu-SAR-bisPSMA for the treatment of mCRPC patients. The first 3 cohorts in the dose escalation phase of the trial were successfully completed with no dose limiting toxicities (DLTs) reported in any of the participants dosed (15 participants). No adverse events (AEs) related to 64Cu-SAR-bisPSMA were observed. Most AEs related to 67Cu-SAR-bisPSMA were low grade (grade 1 or 2). The most common AE reported was mild dry mouth (grade 1, 5/15 participants, 33.3%).
Preliminary data shows that the majority of participants in the SECuRE study enrolled to date had bone metastasis (77%), high median prostate-specific antigen (PSA) level at baseline (112.86 ng/mL, range 0.1-1503.1) and were heavily pre-treated (59% of participants received 3 or more lines of therapy). Despite how heavily pre-treated these participants were, and how much disease they had, 73% of them across all cohorts (including the lowest dose cohort of 67Cu-SAR-bisPSMA at 4 GBq, single dose) showed reductions in PSA levels. The majority of patients that had an increase in PSA were on the single, lowest dose cohort 1 (4 GBq). PSA reductions of greater than 50% were seen in 45% of all trial participants, despite the overwhelming majority of participants only receiving a single dose of 67Cu-SAR-bisPSMA (4, 8 or 12 GBq) in the trial. In cohorts 2, 3 and 4 (8 and 12 GBq single dose and 12 GBq multi-dose, respectively), in which most participants also only received 1 dose of 67Cu-SAR-bisPSMA, PSA reductions of greater than 35% were observed in almost 75% of participants and PSA was reduced by 80% or more in almost half of the participants so far, as patients continue in follow-up.
The trial is currently progressing through the highest dose cohort where participants were administered multiple doses of 12 GBq of 67Cu-SAR-bisPSMA. Recruitment into cohort 4 is complete, and the remaining 3 participants are currently in the safety and efficacy follow-up period after receiving their first 2 doses. Following completion of the follow-up up period, the safety review committee meeting is planned for March 2025. The largest drop in prostate-specific antigen (PSA) in cohort 4 to date is a decline of 98% (from a baseline of 157.4 ng/mL). This participant, who had failed multiple lines of therapy prior to receiving 67Cu-SAR-bisPSMA (androgen deprivation therapy [ADT], ARPI and an investigational agent), has already had a radiographic partial response based on the investigator’s assessment of Response Evaluation Criteria in Solid Tumours v1.1 (RECIST) criteria. Preliminary analysis showed a reduction of 60.6% in tumour volume evaluated by PSMA positron emission tomography [PET] imaging with 64Cu-SAR-bisPSMA (Figure 1).
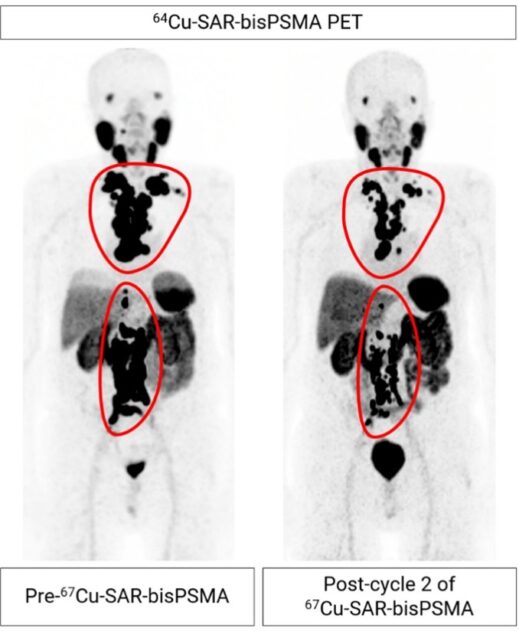
Figure 1. mCRPC patient from cohort 4 showing extensive metastasis of prostate cancer to the lymph nodes (regions highlighted by the red lines). Considerable reduction in tumour volume (60.6%) observed following 2 doses of 67Cu-SAR-bisPSMA (PSMA-avid tumour burden reduction assessed by 64Cu-SAR-bisPSMA PET). Images shown as maximum intensity projections.
Cohort expansion of the SECuRE trial to assess the combination of 67Cu-SAR-bisPSMA with enzalutamide
For a long time, Clarity has been working closely with many important global medical experts in the field of prostate cancer, including the Company’s Clinical Advisory Board members, Prof Louise Emmett and Prof Oliver Sartor, to optimise the development of all of its products in prostate cancer. Those discussions have led to a recent protocol amendment for the SECuRE trial, which aims to investigate ways to further improve the treatment outcomes for these patients. The protocol amendment is aligned with the positive results of the Enza-p trial presented by Prof Emmett first at the European Society for Medical Oncology in 20235 and more recently at the American Society of Clinical Oncology Genitourinary Cancers (ASCO GU) Symposium in 20256, which confirmed the hypothesis that targeting both androgen signalling and PSMA receptors concurrently would improve anti-cancer activity in mCRPC. The latest SECuRE protocol amendment increased the number of participants in the cohort expansion phase from 14 to 24 patients in the mCRPC pre-chemotherapy setting, with a subset of patients to receive the combination therapy of 67Cu-SAR-bisPSMA with enzalutamide. This protocol amendment has now been approved at many of the participating trial sites, and the changes are expected to further enhance the already positive results of 67Cu-SAR-bisPSMA observed in the SECuRE trial to date. This strategy focuses on the commercialisation of the product firstly in the largest market for prostate cancer therapies in mCRPC, with pre-chemotherapy being 3 times larger than the post-chemotherapy setting, and creates opportunities for the use of 67Cu-SAR-bisPSMA with a range of ARPIs in future clinical development. Addressing the radiopharmaceuticals supply issues with current treatments in such a large indication requires a streamlined approach for which Clarity’s 64Cu/67Cu platform is well and uniquely suited.
Clarity’s Executive Chairperson, Dr Alan Taylor, commented, “Receiving 3 FTDs for the one molecule, SAR-bisPSMA, within the last 6 months is an incredible achievement for Clarity, highlighting how impressive our science and development are, the significance of the diagnostic and therapeutic data so far, and the high unmet need for better therapies and diagnostics in prostate cancer.
“The dual-targeted bisPSMA molecule was developed at the benchtop of Australian science with the intent of overcoming the shortfalls of the current generation of PSMA-targeting products. It was optimised with two PSMA ligands, which increases not only the amount of product in the lesions, but also how long the product is retained in the lesions over time, making it an ideal candidate for both diagnosis and therapy. The clinical data in both diagnostic and therapeutic indications that we are generating is remarkable, confirming the results that we initially saw in preclinical development. The granting of FTDs by the US FDA for 3 distinct indications in prostate cancer that we are aiming to address with this product is testament to the incredible work of our team and collaborators. This latest FTD will allow us flexibility to develop 67Cu-SAR-bisPSMA in both pre- and post-chemotherapy patients in the mCRPC setting, with initial focus on the largest market segment. The SECuRE study will also provide invaluable information on the potential of 67Cu-SAR-bisPSMA to be combined with enzalutamide and other ARPIs in future, creating opportunities for the broader use of 67Cu-SAR-bisPSMA in those patients with such high unmet medical need.
“These designations will allow us to work closely with the FDA to facilitate the development process and accelerate the approval of what could become best-in-class therapy and diagnostic agents, and our team and collaborators are committed to making this our priority in order to achieve our ultimate goal of improving treatment outcomes for people with cancer.”

From left to right: A/Prof Sophie Scott (author and broadcaster), Dr Alan Taylor (Executive Chairperson, Clarity Pharmaceuticals), Prof Louise Emmett (Director Theranostics and Nuclear Medicine, St Vincent’s Hospital Sydney) and Prof Oliver Sartor (Chief, Genitourinary Cancers Disease Group and Director Radiopharmaceutical Trials, Mayo Clinic, USA). Theranostics in Focus: New Frontiers in Cancer Care. 4 Feb 2025, Sydney – Australia.
About SAR-bisPSMA
SAR-bisPSMA derives its name from the word “bis”, which reflects a novel approach of connecting two PSMA-targeting agents to Clarity’s proprietary sarcophagine (SAR) technology that securely holds copper isotopes inside a cage-like structure, called a chelator. Unlike other commercially available chelators, the SAR technology prevents copper leakage into the body. SAR-bisPSMA is a Targeted Copper Theranostic (TCT) that can be used with isotopes of copper-64 (Cu-64 or 64Cu) for imaging and copper-67 (Cu-67 or 67Cu) for therapy.
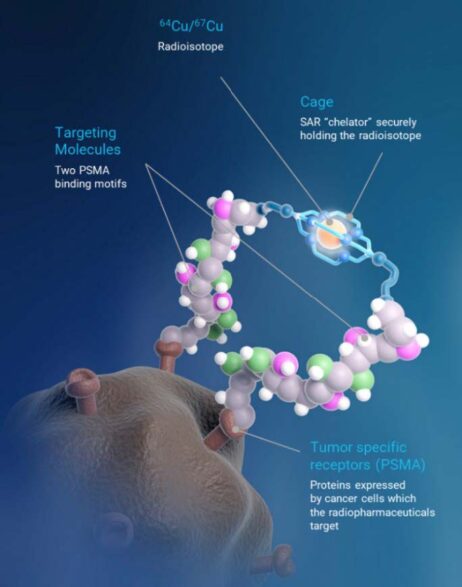
64Cu-SAR-bisPSMA is an unregistered product. The safety and efficacy of 64Cu-SAR-bisPSMA has not been assessed by health authorities such as the U.S. FDA or the Therapeutic Goods Administration (TGA). There is no guarantee that this product will become commercially available.
Overview of Clarity’s SAR-bisPSMA clinical program
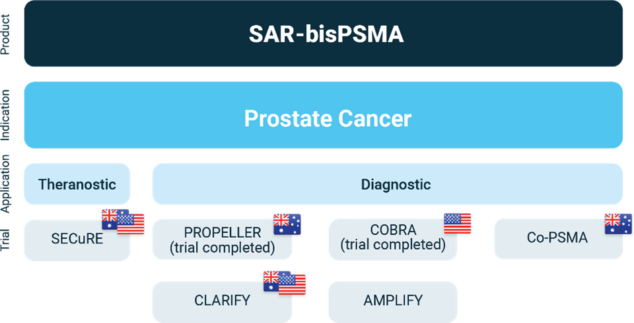
About the SECuRE trial
SECuRE (NCT04868604)4 is a Phase I/IIa theranostic trial for identification and treatment of an advanced form of prostate cancer, mCRPC. It is a multi-centre, single arm, dose escalation study with a cohort expansion. The aim of this trial is to determine the safety and tolerability of both 64Cu-SAR-bisPSMA and 67Cu-SAR-bisPSMA, as well as the efficacy of 67Cu-SAR-bisPSMA as a therapy. A recent protocol amendment has increased the number of participants in the cohort expansion phase from 14 to 24, and a subset of participants will receive the combination of 67Cu-SAR-bisPSMA with enzalutamide.
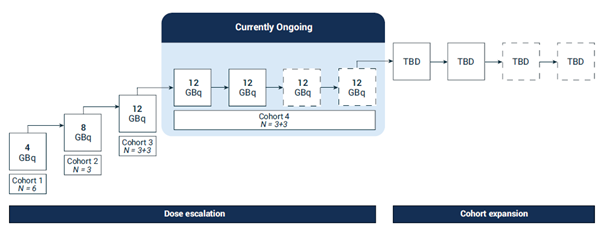
Figure 3. SECuRE Study Design.
About Prostate Cancer
Prostate cancer is the second most common cancer diagnosed in men globally and the fifth leading cause of cancer death in men worldwide7. Prostate cancer is the second-leading causes of cancer death in American men. The American Cancer Institute estimates in 2025 there will be about 313,780 new cases of prostate cancer in the U.S. and around 35,770 deaths from the disease8.
About Clarity Pharmaceuticals
Clarity is a clinical stage radiopharmaceutical company focused on the treatment of serious diseases. The Company is a leader in innovative radiopharmaceuticals, developing Targeted Copper Theranostics based on its SAR Technology Platform for the treatment of cancers in children and adults.
References
- Clarity Pharmaceuticals. Clarity receives FDA Fast Track Designation for 64Cu-SAR-bisPSMA. https://www.claritypharmaceuticals.com/news/fast-track/
- Clarity Pharmaceuticals. Clarity receives U.S. FDA Fast Track Designation for 64Cu-SAR-bisPSMA in biochemical recurrence of prostate cancer. https://www.claritypharmaceuticals.com/news/ftd-2/
- ClinicalTrials.gov Identifier: NCT06056830, https://clinicaltrials.gov/study/NCT06056830
- ClinicalTrials.gov Identifier: NCT04868604, https://clinicaltrials.gov/ct2/show/NCT04868604
- Emmett et al. Enzalutamide and 177Lu-PSMA-617 in poor-risk, metastatic, castration-resistant prostate cancer (mCRPC): A randomised, phase II trial: ENZA-p (ANZUP 1901). Annals of Oncology, Volume 34, S1325, 2023.
- Emmett et al. Overall survival and quality of life with [177Lu] Lu-PSMA-617 plus enzalutamide versus enzalutamide alone in poor-risk, metastatic, castration-resistant prostate cancer in ENZA-p (ANZUP 1901). American Society of Clinical Oncology Genitourinary Cancers (ASCO GU) Symposium, 2025
- Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries, https://acsjournals.onlinelibrary.wiley.com/doi/10.3322/caac.21834
- American Cancer Society: Key Statistics for Prostate Cancer, https://www.cancer.org/cancer/prostate-cancer/about/key-statistics.html
Media Contacts
Clarity Pharmaceuticals
Dr Alan Taylor
Executive Chairperson
ataylor@claritypharm.com
Sodali
Catherine Strong
Investor/Media Relations
+61 (0)406 759 268
catherine.strong@sodali.com
This announcement has been authorised for release by the Executive Chairperson.
