Sydney, Australia 18 December 2024
Highlights
- Clarity has developed a proprietary fibroblast activation protein (FAP)-targeted radiopharmaceutical product that can be used with the perfect pairing of copper isotopes for the diagnosis and treatment of cancer.
- The product, termed SAR-bisFAP, has shown strong tumour targeting, retention and pharmacokinetic data to date in pre-clinical models.
- FAP is expressed widely across a range of malignancies, opening a very large pan-cancer opportunity for both imaging and treatment of various cancers.
- Clarity continues to develop a range of novel products through its Discovery Program with strong patent protection aimed at improving treatment outcomes in indications with high unmet needs.
Clarity Pharmaceuticals (ASX: CU6) (“Clarity” or “Company”), a clinical-stage radiopharmaceutical company with a mission to develop next-generation products that improve treatment outcomes for children and adults with cancer, is pleased to announce the expansion of its pipeline with a novel FAP-targeted radiopharmaceutical for the diagnosis and treatment of cancer.
FAP is expressed on cancer associated fibroblasts (CAFs), a particular cell type found in the tumour microenvironment (cancer ‘infrastructure’ called the tumour stroma). CAFs are found in a broad range of cancers (e.g. breast, colorectal, pancreatic, lung, brain and ovarian cancers), but only minimally in normal tissue, making FAP a promising pan-cancer target for both imaging and treatment of cancers1. CAFs form part of the environment surrounding the cancer cells, and they can promote cancer growth and the spread of the tumour throughout the body2. Targeting the tumour stroma is an alternative way to treat cancer whereby the architecture of the tumour mass is targeted rather than the tumour cells directly.
Clarity’s Targeted Copper Theranostic (TCT) targeting FAP was developed at the benchtop of Australian science, with a clear understanding of other FAP-targeted radiopharmaceuticals in development and the intent of overcoming the low uptake and retention of these agents in tumours. This was achieved by utilising some novel chemistry, and by combining an industry leading FAP inhibitor with the proprietary SAR chelator technology. The SAR Technology enables the use of copper-64 (64Cu) for imaging and copper-67 (67Cu) for the targeted treatment of various cancers.
Similar to how Clarity developed its PSMA-targeted prostate cancer agent as a dimer, SAR-bisPSMA, which was designed to improve tumour uptake and retention, the Company created a novel dimer for its FAP-targeted radiopharmaceutical, SAR-bisFAP. With the benefit of comparing this novel molecule to other FAP radiopharmaceuticals in development as well as to a monomer equivalent (SAR-monoFAP), the dimer SAR-bisFAP has shown increased tumour uptake and retention over 24 hours in pre-clinical models.
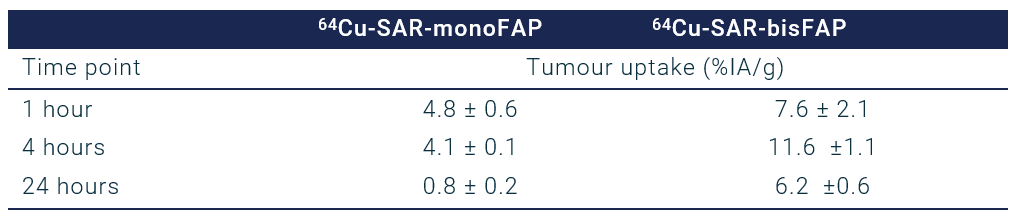
Table 1. Biodistribution of 64Cu-SAR-monoFAP or 64Cu-SAR-bisFAP in a pre-clinical cancer model. In a pre-clinical cancer model utilising a FAP-expressing glioblastoma cell line (U87MG), the biodistribution of 64Cu-SAR-monoFAP and 64Cu-SAR-bisFAP were assessed. The Table shows measurements of how much of the products accumulated in the cancer, which is expressed as the percentage of the injected activity (%IA/g) at either 1, 4, or 24 hours post-injection. The monomer had moderate uptake at 1 hour, which decreased over 24 hours. The dimer had a higher uptake at 1 hour, rising further to 11.6 %IA/g at 4 hours. At 24 hours, the dimer had 6.2 %IA/g, which is approximately 8 times greater retention than the monomer.
In addition to comparing the mono and dimer versions of the product, Clarity compared the dimer, 64Cu-SAR-bisFAP, to an industry standard FAP-targeted monomer called 68Ga-FAPI-46. Using a FAP-expressing melanoma cell line (SK-MEL187) in this experiment, at 1-hour post-injection 64Cu-SAR-bisFAP had approximately 4 times the uptake in the cancer compared to 68Ga-FAPI-46. The improvements in uptake and retention of 64/67Cu-SAR-bisFAP compared to first-generation FAP compounds, such as FAPI-46, are key attributes for the development of next-generation radiopharmaceuticals.
Clarity is currently conducting additional investigations to enable a Phase I clinical trial, which could commence in late 2025. Research into the potential clinical use of Clarity’s FAP agent has begun with several pre-clinical studies in diagnostics (utilising 64Cu-SAR-bisFAP), which will be followed by exploring treatment opportunities of cancers based on their unmet medical needs (using 67Cu-SAR-bisFAP).
Clarity’s Executive Chairperson, Dr Alan Taylor, commented, “Our commitment to always putting science first at Clarity has placed us in an enviable position in radiopharmaceuticals globally. This has allowed us, yet again, to create a novel product at the benchtop to overcome the shortcomings of competing radiopharmaceuticals by increasing the uptake and retention of the molecule over time. Coupled with the use of the perfect pairing of copper isotopes, this facilitates the use of same-day and next-day imaging, addressing the issue of low sensitivity of short half-life products using gallium-68 and fluorine-18, as well as potentially enhancing the therapeutic benefit through increasing the amount and retention of the product at the site of tumours. This is especially the case for FAP-targeted radiopharmaceuticals that offer so much hope as a pan-cancer but suffer the issue of low uptake and retention at the tumour site.
“We are excited to continue growing our pipeline of TCTs through our Discovery Program, utilising the unique advantages of copper isotopes, enabled by our proprietary SAR Technology. Unlike other chelator technologies that leak copper in vivo, the SAR Technology securely holds copper over time, unlocking a myriad of advantages of the “perfect pairing” of copper-64 for imaging and copper-67 for therapy, such as next-day imaging, supply, logistical and environmental advantages. Having strong intellectual property around the SAR Technology, as well as our novel products, with over 28 patent families now within the Company, we continue expanding our pipeline of next-generation radiopharmaceuticals. The development of these new products is only possible due to the utilisation of great chemistry combined with new promising targets and our proprietary chelator, thereby enabling a multitude of new products for indications with high unmet needs. By going back to the drawing board and conducting comprehensive research and testing, we were able to create a unique product that achieves the outcomes we were looking for of improving uptake and retention in tumours. The tumour targeting, retention and pharmacokinetic data we have seen to date with SAR-bisFAP is impressive, and we look forward to progressing this product in clinical trials and are excited to explore the pan-cancer targeting potential in a range of indications with high unmet needs.”
About 64/67Cu-SAR-monoFAP and 64/67Cu-SAR-bisFAP
64/67Cu-SAR-monoFAP and 64/67Cu-SAR-bisFAP are unregistered products. Their safety and efficacy have not been assessed by health authorities such as the US Food and Drug Administration (FDA) or the Therapeutic Goods Administration (TGA). Outcomes from human clinical trials may differ from pre-clinical findings. There is no guarantee that these products will become commercially available.
About Clarity Pharmaceuticals
Clarity is a clinical stage radiopharmaceutical company focused on the treatment of serious diseases. The Company is a leader in innovative radiopharmaceuticals, developing Targeted Copper Theranostics based on its SAR Technology Platform for the treatment of cancers in children and adults.
References
- Xin L, Gao J, Zheng Z, Chen Y, Lv S, Zhao Z, Yu C, Yang X and Zhang R (2021) Fibroblast Activation Protein-α as a Target in the Bench-to-Bedside Diagnosis and Treatment of Tumors: A Narrative Review. Front. Oncol. 11:648187. doi: 10.3389/fonc.2021.648187
- Kwa, M.Q., Herum, K.M. & Brakebusch, C. Cancer-associated fibroblasts: how do they contribute to metastasis?. Clin Exp Metastasis 36, 71–86 (2019). https://doi.org/10.1007/s10585-019-09959-0
Media Contacts
Clarity Pharmaceuticals
Dr Alan Taylor
Executive Chairperson
ataylor@claritypharm.com
Sodali
Catherine Strong
Investor/Media Relations
+61 (0)406 759 268
catherine.strong@sodali.com
This announcement has been authorised for release by the Executive Chairperson.
