Sydney, Australia 28 November 2024
Clarity Pharmaceuticals (ASX: CU6) (“Clarity”), a clinical stage radiopharmaceutical company with a mission to develop next-generation products that improve treatment outcomes for children and adults with cancer, is pleased to announce that the last patient has completed their final assessment in the Phase II diagnostic 64Cu-SARTATE trial, DISCO (NCT04438304)1, for patients with known or suspected neuroendocrine tumours (NETs).
DISCO, which derives from “Diagnostic Imaging Study of 64COpper-SARTATE Using PET on Patients with Known or Suspected Neuroendocrine Tumours”, is assessing the performance of Clarity’s SARTATE imaging product as a potential new method to diagnose and manage NETs. The DISCO trial recruited participants with Gastroenteropancreatic NETs (GEP-NETs) across four sites in Australia, comparing the diagnostic performance of 64Cu-SARTATE at approximately 4 hrs and 20 hrs post-administration to 68Ga-DOTATATE at one hour.
The trial was originally planned for up to 63 patients based on an expected discordance level between imaging with Clarity’s 64Cu-SARTATE and the current standard of care, 68Ga-DOTATATE. The sample size was adjusted to 45 patients based on the results of the pre-planned early assessment of the images collected during the trial with the aim of generating sufficient evidence to plan for a Phase III trial in this indication. This enabled recruitment to successfully close early.
The trial aims to build on earlier work with SARTATE in patients with NETs, which demonstrated that imaging at later time points, enabled by the longer half-life of copper-64 in comparison to gallium-68, may lead to better identification of disease2. Delayed imaging (at 4 hrs and 24 hrs vs 1 hr) showed a progressive increase in lesion-to-liver ratio (Table 1). Figure 1 provides an example of improved lesion detection based on an increase in lesion-to-background ratio observed with delayed imaging2.
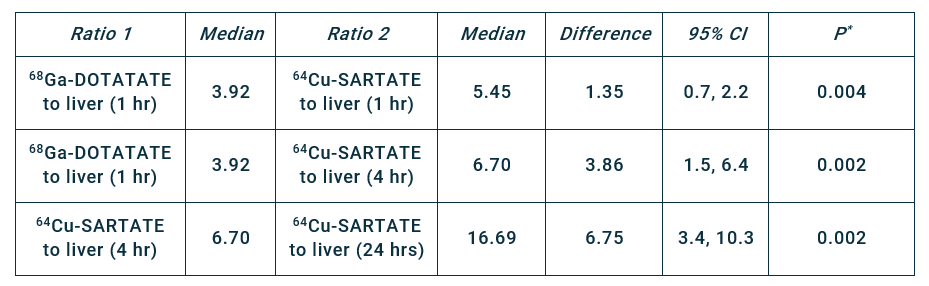
Table 1. Comparison of lesion-to-liver ratios of 68Ga-DOTATATE and 64Cu-SARTATE. Progressive increase in lesion-to-liver ratio with delayed imaging using 64Cu-SARTATE. *Paired Wilcoxon test on 10 patients. CI = confidence interval.
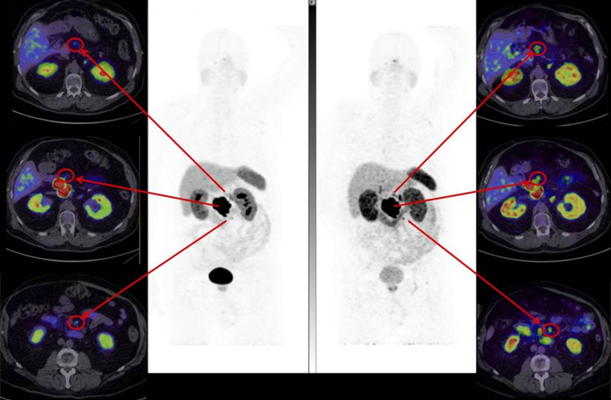
Figure 1. Lesion detection comparing 68Ga-DOTATATE and 64Cu-SARTATE. Hicks et al. (2019) determined superior lesion detection at 4 hrs with 64Cu-SARTATE. High lesion contrast on 64Cu-SARTATE images at 4 hrs (right) better defines regional nodal disease than 68Ga-DOTATATE images at 1 hr (left) in patient with large pancreatic primary tumour.
Clarity’s Executive Chairperson, Dr Alan Taylor, commented, “With the recent changes to the U.S. Centers for Medicare and Medicaid Services (CMS) and reimbursement of radio-diagnostics, this market is set to surge. The market opportunity for each radiodiagnostic is now massive, but there are limitations to the current range of products, most notably, the use of short half-life isotopes and short shelf-life products.
“Imaging at a one-hour time point due to the isotope half-life or product shelf-life, as opposed to patient needs, significantly impacts patient care. Firstly, the scheduling of patients may represent a challenge due to the lack of flexibility for imaging. But importantly, later time-point imaging presents significant benefits for doing what cancer diagnostics need to do, and that is finding cancer. At Clarity, we have known this for many years and have demonstrated these benefits time and time again with different products in our Targeted Copper Theranostic (TCT) platform, including SARTATE. We have seen first-hand in clinical trials that once these radiopharmaceutical products are administered, they take time to find the lesion whilst also needing to clear from background organs, providing greater contrast, especially to identify more difficult to find cancers. This is known as signal-to-noise ratio or, in our case, tumour-to-background ratio. We have also seen to date that these physiological characteristics of radiopharmaceutical products in finding the tumour and clearing the background are not overcome with the small number of state-of-the-art whole body positron emission tomography (PET) imaging cameras, despite their increases in sensitivity.
“Copper-64 has comparably low radiation doses to currently used isotopes and involves similar management of patients. However, the longer half-life of copper-64 of 12.7 hours, combined with Clarity’s proprietary position in copper-based theranostics, now sets up a foundation for a complete suite of next-generation radiodiagnostics, which could be unmatched in the radiopharmaceutical sector. The opportunity for centralised, multi-layer manufacturing and broad distribution means that every patient with access to PET imaging can potentially have optimal cancer diagnostic care, not limited by isotope half-life.
“We have adopted a dual strategy to date with the SARTATE product. Our initial focus is on the theranostic development of 64/67Cu-SARTATE for neuroblastoma in children with the CL04 trial currently ongoing (NCT04023331)3. Clarity secured two Rare Pediatric Disease Designations (RPDD) and two Orphan Drug Designations (ODD) in this indication. Should Clarity be successful in achieving marketing approval from the U.S. Food and Drug Administration (FDA) for these two products in neuroblastoma, RPDDs may allow the Company to access a total of two tradeable Priority Review Vouchers (PRVs) currently valued at around US$158 million each4. Our second strategy is the diagnostic development of 64Cu-SARTATE in a larger market of NETs and other diseases that express the somatostatin receptor 2 (SSTR2) target, followed by therapy in these same indications. We expect the final results from the DISCO study to be available in the first half of 2025, which will inform the design of our registrational Phase III trial in this indication.
“Patients with NETs are often misdiagnosed and experience delays in receiving the correct diagnosis, which leads to disease progression and identifying the cancer at its later stages. We believe that SARTATE has the potential to become a best-in-class product in this indication, and other indications that have the same target receptor, SSTR2, playing an important role in improving accurate staging, lesion identification and treatment outcomes for these patients. The market for these diagnostics in the United States alone is currently in the hundreds of millions of dollars with products that were commercialised prior to the CMS changes. Our team has been focusing on significantly increasing our diagnostic platform to benefit from our proprietary position and the change in market dynamics for radiodiagnostics brought by the CMS. We think that the advantages of copper-64 based on the potential of a flexible dosing schedule, later time-point imaging and logistical benefits of Clarity’s copper-based products could ensure timely diagnosis and enable broader access to critical imaging products for cancer patients around the world.”
About SARTATE
SARTATE is a next generation, highly targeted theranostic radiopharmaceutical. It is being developed for diagnosing, staging and subsequently treating cancers that express somatostatin receptor 2 (SSTR2), including neuroblastoma and neuroendocrine tumours (NETs). Like all Clarity products, the SARTATE product can be used with copper-64 (64Cu) for imaging (64Cu-SARTATE) or copper-67 (67Cu) for therapy (67Cu-SARTATE).
64Cu-SARTATE and 67Cu-SARTATE are unregistered products. Their safety and efficacy have not been assessed by health authorities such as the US Food and Drug Administration (FDA) or the Therapeutic Goods Administration (TGA). Individual results may not represent the overall safety and efficacy profiles of the products. There is no guarantee that these products will become commercially available.
Overview of Clarity’s SARTATE clinical program
About NETs
NETs, also known as well-differentiated neuroendocrine neoplasms or carcinoids, represent a heterogeneous group of malignant transformations of cells of the diffuse neuroendocrine system5. They most commonly occur in the gastrointestinal tract (48%), lung (25%), and pancreas (9%), but may also originate in other areas, including the breast, prostate, thymus and skin6. NETs can either be benign or malignant, as well as non-functional and functional7. NETs traditionally have been considered uncommon; however, the incidence has been increasing as a worldwide phenomenon8.
Overall, it is estimated that more than 12,000 people in the United States are diagnosed with a NET each year, and approximately 170,000 people are living with this diagnosis9. Patients with NETs present with subtle clinical symptoms, which can lead to a delay in diagnosis of more than 4 years10. As such, about 30-75% of NET patients have distant metastases at the time of diagnosis11. A 10-year relative survival rate for patients with metastatic GEP-NETs is 3–36%12.
About Clarity Pharmaceuticals
Clarity is a clinical stage radiopharmaceutical company focused on the treatment of serious disease. The Company is a leader in innovative radiopharmaceuticals, developing targeted copper theranostics based on its SAR Technology Platform for the treatment of cancer in children and adults.
References
- Clinicalstrials.gov Identifier: NCT04438304 https://clinicaltrials.gov/ct2/show/NCT04438304
- Hicks R et al. First-in-human trial of 64Cu-SARTATE PET imaging of patients with neuroendocrine tumours demonstrates high tumor uptake and retention, potentially allowing prospective dosimetry for peptide receptor radionuclide therapy. The Journal of Nuclear Medicine. 2019.
- Clinicaltrials.gov Identifier: NCT04023331 https://www.clinicaltrials.gov/study/NCT04023331
- Brennan, Z. (2024, 30 August). Ipsen sells priority review voucher for highest price since 2016. com/ipsen-sells-priority-review-voucher-for-highestprice-since-2016/
- Cheung VTF, Khan MS. A guide to midgut neuroendocrine tumours (NETs) and carcinoid syndrome. Frontline gastroenterology. 2015;6(4):264-269.
- Hallet J, Law CH, Cukier M, Saskin R, Liu N, Singh S. Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer. 2015;121(4):589-597.
- Yau H, Kinaan M, Quinn SL, Moraitis AG. Octreotide long-acting repeatable in the treatment of neuroendocrine tumors: patient selection and perspectives. Biologics : targets & therapy. 2017;11:115-122.
- Leoncini E, Boffetta P, Shafir M, Aleksovska K, Boccia S, Rindi G. Increased incidence trend of low-grade and high-grade neuroendocrine neoplasms. Endocrine. 2017 Nov;58(2):368-379. doi: 10.1007/s12020-017 1273-x. Epub 2017 Mar 16. PMID: 28303513; PMCID: PMC5671554.
- Types of Neuroendocrine Tumors, Abramson Cancer Center, Penn Medicine, https://www.pennmedicine.org/cancer/types-of-cancer/neuroendocrine-tumors/types-of-neuroendocrine-tumors#:~:text=Experience%20With%20the%20Full%20Range,know%20all%20the%20reasons%20yet.
- Basuroy R, Bouvier C, Ramage JK, Sissons M, Srirajaskanthan R. Delays and routes to diagnosis of neuroendocrine tumours. BMC Cancer. 2018 Nov 16;18(1):1122. doi: 10.1186/s12885-018-5057-3. PMID: 30445941; PMCID: PMC6240263.
- Aluri V. and Dillion, J.S. 2017, “Biochemical Testing in Neuroendocrine Tumors”, Endocrinology & Metabolism Clinics of North America, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5777173/
- Polee, I.N. et al. 2022, “Long-term survival in patients with gastroenteropancreatic neuroendocrine neoplasms: A population-based study”, European Journal of Cancer, Volume 172, 2022, Pages 252-263, ISSN 0959-8049, https://doi.org/10.1016/j.ejca.2022.06.003.
- Krasnovskaya et al. Recent Advances in 64Cu/67Cu-Based Radiopharmaceuticals. Int J Mol Sci. 2023 May 23;24(11):9154. doi: 10.3390/ijms24119154.
- Data on file.
Media Contacts
Clarity Pharmaceuticals
Dr Alan Taylor
Executive Chairperson
ataylor@claritypharm.com
Sodali
Catherine Strong
Investor/Media Relations
+61 (0)406 759 268
catherine.strong@sodali.com
This announcement has been authorised for release by the Executive Chairperson.
