Sydney, Australia 27 October 2022
Clarity Pharmaceuticals (ASX: CU6) (“Clarity”), a clinical-stage radiopharmaceutical company with a mission to develop next-generation products that improve treatment outcomes for children and adults with cancer, is pleased to announce its diagnostic 64Cu SAR-bisPSMA trial (COBRA NCT052491271) for patients with prostate cancer has reached the fifty percent recruitment milestone, with 25 out of 50 participants now having been enrolled and imaged.
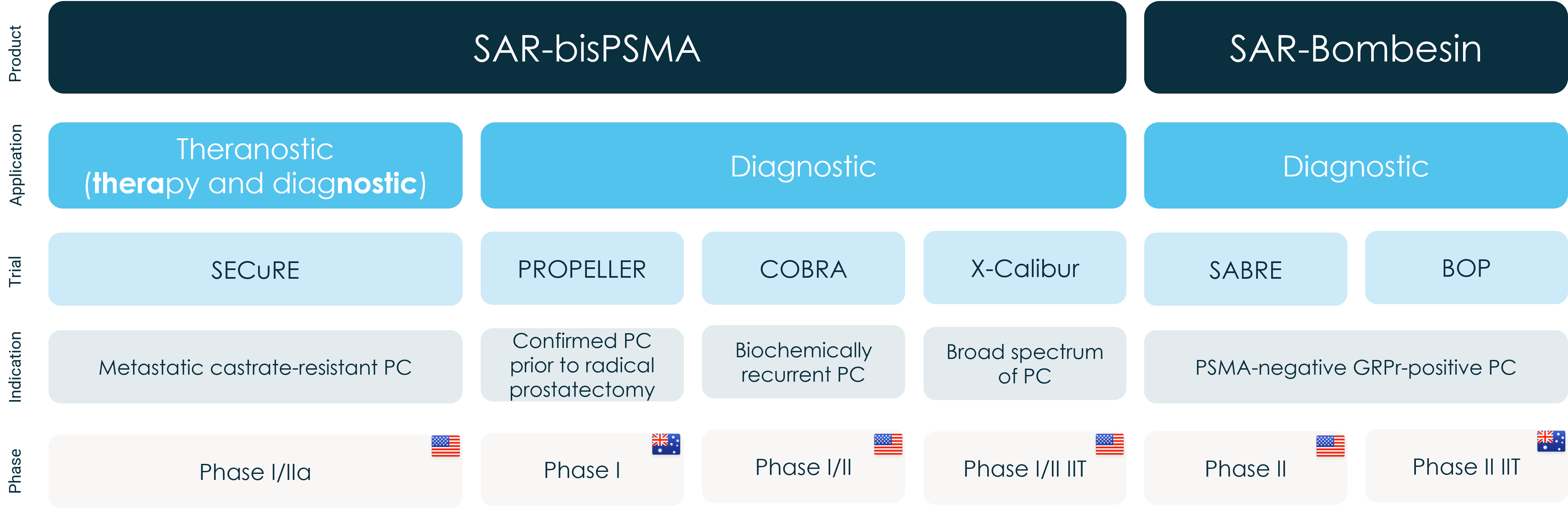
COBRA (Copper-64 SAR-BisPSMA in Biochemically Recurrent prostAte cancer) is a Phase I/II Positron Emission Tomography (PET) trial of participants with biochemical recurrence (BCR) of prostate cancer following definitive therapy. It is a multi-centre, single arm, non-randomised, open-label trial of 64Cu-labelled SAR-bisPSMA in 50 participants. The primary objectives of the trial are to investigate the safety and tolerability of 64Cu-SAR-bisPSMA as well as its ability to correctly detect the recurrence of prostate cancer.
Dr Neal Shore MD, FACS, Lead Principal Investigator in the COBRA trial and CMO – Urology/Surgical Oncology, GenesisCare, US and the Medical Director of Carolina Urologic Research Centre, commented, “We are very pleased with the progress of the COBRA trial, specifically in regard to the pace of recruitment and the quality of data we are generating to explore and validate the clinical benefits associated with the novel SAR-bisPSMA agent. The growing amount of data from Clarity’s three clinical trials with the 64Cu SAR-bisPSMA product, namely, COBRA, PROPELLER and SECuRE trials, all indicate high uptake of the diagnostic agent by prostate cancer cells. This is especially important for patients with suspected prostate cancer recurrence where SAR-bisPSMA shows promise of improving prostate cancer detection.
“We look forward to recruiting the remaining participants in the COBRA trial and commencing the analysis of the study data. Ultimately, we want to enhance diagnostic accuracy for patients with BCR of prostate cancer as well as improve ease of access to the product across the US, enabled by the logistical advantages of Clarity’s Targeted Copper Theranostic platform.”
Clarity’s Executive Chairman, Dr Alan Taylor, commented, “We are excited to have recruited half of the patients planned for the COBRA trial so quickly. We now have 6 sites actively recruiting the remaining participants with suspected recurrence of their prostate cancer across the US.
“Since opening recruitment into the COBRA trial in March 2022, we have been able to generate strong preliminary data and further strengthen and validate our on-demand distribution model of TCTs with all three of our 64Cu-labelled products, SAR-bisPSMA, SARTATE and SAR-Bombesin, being shipped to numerous trial sites in the US from a central manufacturing facility. TCTs have the potential to enable patient access to critical treatments that are safe and efficacious, on time and at any treatment centre with a positron emission tomography camera.
“This shows promise of substantially growing the radiopharmaceutical field into the large oncology market, moving towards the big pharma model with ready-to-use products on-demand and minimising logistical hindrances associated with the current generation of products. We believe this advancement will help us reach our goal of improving treatment outcomes for children and adults with cancer by focusing on the needs of the patients and their treating staff,” said Dr Taylor.
Clarity’s Prostate Cancer clinical trial program overview
About SAR-bisPSMA
SAR-bisPSMA derives its name from the word “bis”, which reflects a novel approach of connecting two prostate-specific membrane antigen (PSMA) binding motifs to Clarity’s proprietary sarcophagene (SAR) technology that securely holds copper isotopes inside a cage-like structure, called a chelator. Unlike other commercially available chelators, the SAR technology prevents copper leakage into the body. SAR-bisPSMA is a Targeted Copper Theranostic (TCT) that can be used with isotopes of copper-64 (Cu-64 or 64Cu) for imaging and copper-67 (Cu-67 or 67Cu) for therapy.
About Prostate Cancer
Prostate cancer is the second most common cancer diagnosed in men globally and the fifth leading cause of cancer death worldwide2. The National Cancer Institute estimates in 2022 there will be 268,490 new cases of prostate cancer in the US and around 34,500 deaths from the disease3.
About Clarity
Clarity is a clinical stage radiopharmaceutical company focused on the treatment of serious disease. The Company is a leader in innovative radiopharmaceuticals, developing targeted copper theranostics based on its SAR Technology Platform for the treatment of cancer in children and adults.
www.claritypharmaceuticals.com
References
- Clinicaltrials.gov Identifier: NCT05249127 <https://clinicaltrials.gov/ct2/show/NCT05249127>
- Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries <https://acsjournals.onlinelibrary.wiley.com/doi/10.3322/caac.21660>
- American Cancer Society, Cancer Statistics Center, <https://cancerstatisticscenter.cancer.org/?_ga=2.79808020.284532473.1620009137-1916069442.1615761164#!/cancer-site/Prostate>
Clarity Pharmaceuticals
Dr Alan Taylor
Executive Chairman
+61 (0)413 871 165
ataylor@claritypharm.com
Citadel-MAGNUS
Catherine Strong
Investor/Media Relations
+61 (0)406 759 268
cstrong@citadelmagnus.com
This announcement has been authorised for release by the Executive Chairman.
