Sydney, Australia 2 November 2022
Clarity Pharmaceuticals (ASX: CU6) (“Clarity”), a clinical-stage radiopharmaceutical company with a mission to develop next-generation products that improve treatment outcomes for children and adults with cancer, is pleased to announce the Phase II diagnostic 64Cu SAR-Bombesin trial (BOP) for patients with prostate cancer has reached the fifty percent recruitment milestone, with 15 out of 30 participants enrolled and imaged.
Prof Louise Emmett (St Vincent’s Hospital Sydney), Principal Investigator in the BOP trial, commented, “We are very excited with the fast pace of recruitment into the BOP trial. We dosed the first patient in mid-September and have reached a 50% recruitment milestone less than 2 months later. The data we are generating will help to explore and validate the clinical benefits of the SAR-Bombesin product. We look forward to recruiting the remaining 15 patients in the trial and analysing the study results.
“We believe SAR-Bombesin will play a role in the identification of disease that is not observed with conventional imaging or PSMA-PET. This could ultimately lead to more effective treatments for this large patient population where unfortunately, very few treatment options are available at present.”
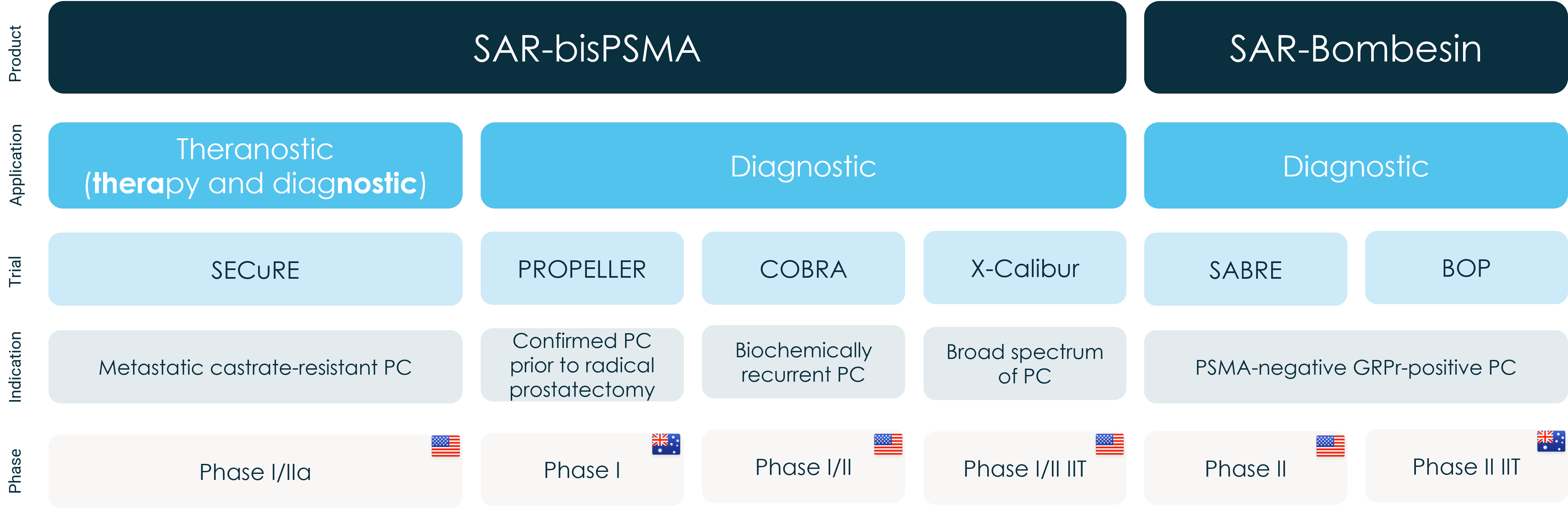
BOP (Copper-64 SAR Bombesin in Prostate Specific Membrane Antigen (PSMA) negative Prostate Cancer) is a Phase II investigator-initiated trial (IIT) in up to 30 patients led by Prof Louise Emmett at St Vincent’s Hospital, Sydney. The BOP trial is assessing the safety of 64Cu-SAR-Bombesin as well as looking at the diagnostic potential across two different groups of men:
- Participants with suspected biochemical recurrence (BCR) of their prostate cancer who have negative PSMA positron emission tomography (PET) imaging scans or low PSMA expression disease.
- Participants with metastatic castrate resistant prostate cancer (mCRPC) who are not eligible for PSMA therapy.
Clarity’s Executive Chairman, Dr Alan Taylor, commented, “The rapid progress of the BOP IIT, led by Prof Emmett and her team at St Vincent’s Hospital, is testament to the hard work and dedication to our mutual goal of improving treatment outcomes for people with cancer. We are motivated and driven by the progress on our collaboration as it has already resulted in improvements to the management of the disease for patients with PSMA-negative prostate cancer.
“The BOP trial builds on the data Prof Emmett’s team generated in PSMA-negative prostate cancer patients imaged under the Therapeutic Goods Administration Special Access Scheme, as well as from the pilot diagnostic trial investigating SAR-Bombesin in breast cancer patients. The data indicates potential utility of the product as a theranostic agent. As such, Clarity plans to progress the therapy under an Investigational New Drug application with the US Food and Drug Administration for the commencement of a theranostic trial with SAR-Bombesin in the US. We believe SAR-Bombesin has the potential to provide large patient populations with accurate and precise detection and treatment of cancers.”
Clarity’s Prostate Cancer clinical trial program overview
About SAR-Bombesin
SAR-Bombesin is a highly targeted pan-cancer radiopharmaceutical with broad cancer application. It targets the gastrin-releasing peptide receptor (GRPr) present on cells of a range of cancers, including but not limited to prostate, breast and ovarian cancers. GRPr is found in approximately 75-100% of prostate cancers, including prostate cancers that don’t express PSMA (PSMA-negative)1-5. The product utilises Clarity’s proprietary sarcophagine (SAR) technology that securely holds copper isotopes inside a cage-like structure, called a chelator. Unlike other commercially available chelators, the SAR technology prevents copper leakage into the body. SAR-Bombesin is a Targeted Copper Theranostic (TCT) that can be used with isotopes of copper-64 (Cu-64 or 64Cu) for imaging and copper-67 (Cu-67 or 67Cu) for therapy.
About Prostate Cancer
Prostate cancer is the second most common cancer diagnosed in men globally and the fifth leading cause of cancer death worldwide6. The National Cancer Institute estimates in 2022 there will be 268,490 new cases of prostate cancer in the US and around 34,500 deaths from the disease7.
Approximately 20% of prostate cancers with BCR are PSMA-PET negative8-11. These patients are therefore unlikely to respond to therapeutic PSMA-targeted products and currently have few treatment options available to them. Given the prostate cancer indication is one of the largest in oncology, there is a significant unmet medical need in this segment.
About Clarity
Clarity is a clinical stage radiopharmaceutical company focused on the treatment of serious disease. The Company is a leader in innovative radiopharmaceuticals, developing targeted copper theranostics based on its SAR Technology Platform for the treatment of cancer in children and adults.
www.claritypharmaceuticals.com
References
- Markwalder R, Reubi JC. Gastrin-releasing peptide receptors in the human prostate: relation to neoplastic transformation. Cancer research. 1999;59(5):1152-1159.
- Fleischmann A, Waser B, Reubi JC. High expression of gastrin-releasing peptide receptors in the vascular bed of urinary tract cancers: promising candidates for vascular targeting applications. Endocrine-related cancer. 2009;16(2):623-633.
- Ananias HJ, van den Heuvel MC, Helfrich W, de Jong IJ. Expression of the gastrin-releasing peptide receptor, the prostate stem cell antigen and the prostate-specific membrane antigen in lymph node and bone metastases of prostate cancer. The Prostate. 2009;69(10):1101-1108.
- Reubi JC, Wenger S, Schmuckli-Maurer J, Schaer JC, Gugger M. Bombesin receptor subtypes in human cancers: detection with the universal radioligand (125)I-[D-TYR(6), beta-ALA(11), PHE(13), NLE(14)] bombesin(6-14). Clin Cancer Res. 2002;8(4):1139-1146.
- Sun B, Halmos G, Schally AV, Wang X, Martinez M. Presence of receptors for bombesin/gastrin-releasing peptide and mRNA for three receptor subtypes in human prostate cancers. The Prostate. 2000;42(4):295-303.
- Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries <https://acsjournals.onlinelibrary.wiley.com/doi/10.3322/caac.21660>
- American Cancer Society, Cancer Statistics Center, <https://cancerstatisticscenter.cancer.org/?_ga=2.79808020.284532473.1620009137-1916069442.1615761164#!/cancer-site/Prostate>
- Afshar-Oromieh A, Holland-Letz T, Giesel FL, et al. Diagnostic performance of 68Ga-PSMA-11 (HBED-CC) PET/CT in patients with recurrent prostate cancer: evaluation in 1007 patients. Eur J Nucl Med Mol Imaging. 2017 Aug;44(8):1258-1268.
- Ferraro DA, Rüschoff JH, Muehlematter UJ, et al. Immunohistochemical PSMA expression patterns of primary prostate cancer tissue are associated with the detection rate of biochemical recurrence with 68Ga-PSMA-11-PET. Theranostics. 2020;10(14):6082-6094.
- Baratto L, Song H, Duan H, et al. PSMA- and GRPR-Targeted PET: Results from 50 Patients with Biochemically Recurrent Prostate Cancer. J Nucl Med. 2021;62(11):1545-1549.
- Mapelli P, Ghezzo S, Samanes Gajate AM, et al. 68Ga-PSMA and 68Ga-DOTA-RM2 PET/MRI in Recurrent Prostate Cancer: Diagnostic Performance and Association with Clinical and Histopathological Data. Cancers (Basel). 2022;14(2):334.
Media Contact
Clarity Pharmaceuticals
Dr Alan Taylor
Executive Chairman
+61 (0)413 871 165
ataylor@claritypharm.com
Citadel-MAGNUS
Catherine Strong
Investor/Media Relations
+61 (0)406 759 268
cstrong@citadelmagnus.com
This announcement has been authorised for release by the Executive Chairman.
