Sydney, Australia 17 April 2025
Highlights
- Clarity received an $11,146,204 Research and Development (R&D) Tax Incentive refund, inclusive of interest, as part of the Australian Federal Government’s R&D Tax Incentive program.
- The receipt of the $11.1 million will provide additional funding to further the development of Clarity’s Targeted Copper Theranostic (TCT) platform of products for various cancer indications.
- Clarity is well funded with approximately $106 million in cash, the majority of which will be spent directly on research, development and commercialisation endeavours in the Company’s key programs.
- Following a thorough review of Clarity’s portfolio of clinical-stage assets as well as an in-depth analysis of the markets and their potential risks, the Company will prioritise the development of 64/67Cu-SAR-bisPSMA for both diagnostic and therapeutic applications in prostate cancer as well as the development of 64Cu-SARTATE in neuroendocrine tumours (NETs) and 64Cu-SAR-Bombesin in breast and prostate cancers.
- Clarity will also continue to progress its Discovery Program, aiming to bring key assets such as 64Cu-SAR-bisFAP and 64/67Cu-SAR-trastuzumab to the clinic.
- As part of this priortitisation process, the CL04 trial with 64/67Cu-SARTATE in paediatric high-risk neuroblastoma and the COMBAT trial with 64/67Cu-SAR-Bombesin in low prostate-specific membrane antigen (PSMA) metastatic castration-resistant prostate cancer (mCRPC) will be closed.
Clarity Pharmaceuticals (ASX: CU6) (“Clarity” or “Company”), a clinical-stage radiopharmaceutical company with a mission to develop next-generation products that improve treatment outcomes for patients with cancer, is pleased to announce that it has received an $11,146,204 R&D Tax Incentive refund, including interest, as part of the Australian Federal Government’s R&D Tax Incentive program, recognising the R&D undertaken by Clarity in the radiopharmaceutical field in the financial year ended 30 June 2024.
The Australian Federal Government’s R&D Tax Incentive program encourages companies to engage and invest in R&D activities by providing a refundable tax offset of up to 48.5% of the eligible activities. With the addition of the $11.1 million, and a closing cash balance at the end of March 2025 of approximately $95 million, Clarity is well funded with approximately $106 million in cash, the predominance of which will be spent directly on research, development and commercialisation endeavours in the Company’s key programs.
Clarity’s Executive Chairperson, Dr Alan Taylor, commented, “The R&D Tax Incentive program offered by the Australian Government provides an extremely valuable source of non-dilutive funding for Clarity, delivering significant assistance as we continue to explore applications of our unique SAR Technology to address indications with high unmet needs. While Clarity’s current funds are sufficient to continue pursuing our ultimate goal of improving treatment outcomes for people with cancer, we cannot ignore the recent headwinds in financial markets since the US Presidential election in November 2024. Some of the most prominent and relevant issues include a fall of over 30% in the XBI (US Biotech Index) from its peak in November 2024 to its low in April 2025, as well as corrections in global market indexes, including the S&P500 and NASDAQ in the US, as well as the All Ordinaries and the ASX200 in Australia; changes within the US Food and Drug Administration (FDA); global tariff and trade war concerns and the collapse of Opthea in the local market. Despite being external to the Company, all of these events have had a deleterious effect on our share price. As a result, the Board of Directors of Clarity has taken the view that it is prudent to stretch out the funding runway during this period of volatility by focusing the Company’s strategy on high-value projects and clinical programs that have high probabilities of success and provide early opportunities for commercialisation and to extend our runway into the second half of 2026.”
SAR-bisPSMA
Following a thorough review of Clarity’s portfolio of clinical-stage assets as well as an in-depth analysis of the markets and their potential risks, the Company will prioritise the development of 64/67Cu-SAR-bisPSMA for both diagnostic and therapeutic applications in prostate cancer. SAR-bisPSMA was recently awarded three Fast Track Designations for SAR-bisPSMA1,2,3 based on the data from comparator diagnostic trials versus standard-of-care (SOC) PSMA imaging, as well as the Dose Escalation Phase of the SECuRE trial (NCT04868604)4.
“This optimised asset is the jewel in the crown of Clarity’s strategy and continues to generate exciting data in the ongoing clinical trials. We believe it will entirely disrupt the PSMA-targeted diagnostic and therapeutic markets. These markets, in their entirety, represent a potential size of well in excess of US$10 billion, and we look forward to early commercialisation following completion of Clarity’s CLARIFY (NCT06056830)5 and AMPLIFY registrational trials,” said Dr Taylor.
SARTATE
In addition to the SAR-bisPSMA development, Clarity will prioritise the development of SARTATE into early commercialisation with a focus on NETs imaging in the first instance.
Clarity has had a dual-track approach with SARTATE to date, developing this product in two indications: NETs and a high-risk paediatric cancer, neuroblastoma. Neuroblastoma is an aggressive childhood cancer and, with two Rare Paediatric Disease Designations (RPDDs) awarded to Clarity by the FDA for 64Cu-SARTATE and 67Cu-SARTATE, the Company was eligible to access two tradeable Priority Review Vouchers (PRVs) previously valued at ~$158M USD each6 upon achieving marketing approvals from the FDA. With the risk to the continuation of the PRV program under the current US administration, the changes to the FDA and the very small patient population in neuroblastoma, despite the high unmet need, and the much faster speed we could progress the adult indication of NETs, it was decided to close the CL04 program.
Progressing SARTATE as a diagnosic in NETs is a faster and larger market opportunity for commercialisation. The Phase II DISCO trial (NCT04438304)7 with 64Cu-SARTATE in NETs has successfully closed recruitment with topline data to be announced in the coming months, leading to preparation for a Phase III trial in this patient population. The DISCO trial aims to build on earlier work with SARTATE in patients with NETs, which demonstrated that imaging at later time points, enabled by the longer half-life of copper-64 in comparison to gallium-68, may lead to better identification of disease8.
Development of SARTATE as a therapy will focus on larger market opportunities including NETs, breast cancer and the potential for combination therapies.
SAR-Bombesin
Clarity will progress the development of SAR-Bombesin (SAR-BBN) with a focus on prostate cancer imaging in the first instance.
The Phase II SABRE trial (NCT05407311)9 with 64Cu-SAR-Bombesin in prostate cancer successfully closed recruitment with topline data to be announced in the coming months. The SABRE trial is in the PSMA-negative prostate cancer indication, utilising the current SOC PSMA agents, an area Clarity is also exploring with the optimised SAR-bisPSMA agent.
With regards to the Phase I/IIa theranostic COMBAT trial in prostate cancer with SAR-Bombesin, the Board has chosen to discontinue the program.
“The SAR-bisPSMA therapy program offers so many prostate cancer patients hope and by far massively overshadows the very small population of patients that may benefit from 67Cu-SAR-Bombesin therapy. As such, the Board has decided to redeploy Clarity people and capital to the much greater opportunity for SAR-bisPSMA. SAR-Bombesin will continue to be explored as a diagnostic in prostate cancer at this stage, and as a diagnostic and therapy in a range of other malignancies, including breast cancer.
“We will be working closely with our clinical sites and vendors on activities required to close out the CL04 and COMBAT trials accordingly,” said Dr Taylor.
Clarity will also continue to progress its Discovery Program, aiming to bring key assets, such as 64Cu-SAR-bisFAP10 and 64/67Cu-SAR-trastuzumab11, to the clinic.
Dr Taylor commented, “It has been an incredibly difficult decision to close these 2 theranostic studies, CL04 and COMBAT, and one which has not been made lightly. Importantly, it is not a result of any safety concerns or adverse findings related to their respective products. This step is largely a reflection of the turbulence in the financial markets, necessitating a conservative and focused approach to clinical trial funding. Theranostic trials, such as CL04 and COMBAT, are a significant financial undertaking compared to diagnostic programs, bear a higher degree of risk and have a longer pathway to market. We are disappointed to not progress with these trials and whilst this is not the outcome we wanted, we are confident that this is the right path for Clarity, the team, shareholders and, ultimately, the patients at this time. Our refocus demonstrates strong alignment with Clarity’s core diagnostic and therapeutic strategies, will allow the most efficient allocation of resources and is in line with our long-term value creation objectives and the goal of improving treatment outcomes for cancer patients in need of effective diagnostics and therapies.”
About SAR-bisPSMA
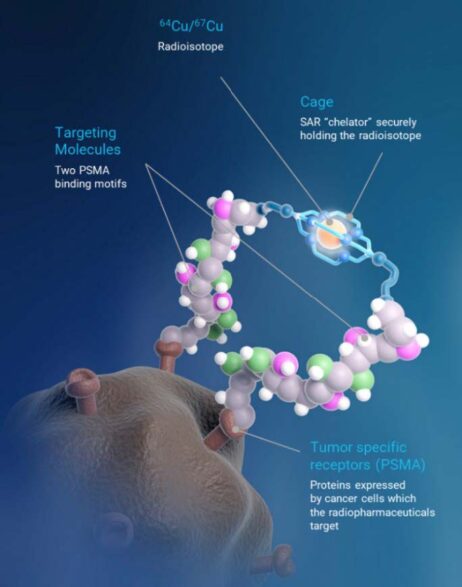
SAR-bisPSMA derives its name from the word “bis”, which reflects a novel approach of connecting two PSMA-targeting agents to Clarity’s proprietary sarcophagine (SAR) technology that securely holds copper isotopes inside a cage-like structure, called a chelator. Unlike other commercially available chelators, the SAR technology prevents copper leakage into the body. SAR-bisPSMA is a Targeted Copper Theranostic (TCT) that can be used with isotopes of copper-64 (Cu-64 or 64Cu) for imaging and copper-67 (Cu-67 or 67Cu) for therapy.
Disclaimer
Clarity’s assets mentioned in this announcement are unregistered products. Their safety and efficacy have not been assessed by health authorities such as the US FDA or the Therapeutic Goods Administration (TGA). There is no guarantee that these products will become commercially available.
About Clarity Pharmaceuticals
Clarity is a clinical stage radiopharmaceutical company focused on the treatment of serious diseases. The Company is a leader in innovative radiopharmaceuticals, developing Targeted Copper Theranostics based on its SAR Technology Platform for the treatment of cancers.
Media Contacts
Clarity Pharmaceuticals
Dr Alan Taylor
Executive Chairperson
ataylor@claritypharm.com
Sodali
Catherine Strong
Investor/Media Relations
+61 (0)406 759 268
catherine.strong@sodali.com
References
- Clarity Pharmaceuticals. Clarity receives FDA Fast Track Designation for Cu-64 SAR-bisPSMA. https://www.claritypharmaceuticals.com/news/fast-track/
- Clarity Pharmaceuticals. Clarity receives U.S. FDA Fast Track Designation for Cu-64 SAR-bisPSMA in biochemical recurrence of prostate cancer. https://www.claritypharmaceuticals.com/news/ftd-2/
- Clarity Pharmaceuticals. Clarity receives US FDA Fast Track Designation for the treatment of metastatic castration-resistant prostate cancer patients with Cu-67 SAR-bisPSMA. https://www.claritypharmaceuticals.com/news/ftd-67cu-sarbispsma
- ClinicalTrials.gov Identifier: NCT04868604, https://clinicaltrials.gov/ct2/show/NCT04868604
- ClinicalTrials.gov Identifier: NCT06056830, https://clinicaltrials.gov/ct2/show/NCT06056830
- Ipsen: Ipsen announces sale of Priority Review Voucher for $158m, www.ipsen.com/statement/ipsen-announces-sale-of-priority-reviewvoucher-for-158m-2935903/
- ClinicalTrials.gov Identifier: NCT04868604, https://clinicaltrials.gov/study/NCT04438304
- Hicks R et al. First-in-human trial of 64Cu-SARTATE PET imaging of patients with neuroendocrine tumours demonstrates high tumor uptake and retention, potentially allowing prospective dosimetry for peptide receptor radionuclide therapy. The Journal of Nuclear Medicine. 2019.
- ClinicalTrials.gov Identifier: NCT05407311, https://clinicaltrials.gov/ct2/show/NCT05407311
- Clarity Pharmaceuticals. Clarity expands its pipeline with a novel optimised FAP-targeted radiopharmaceutical. https://www.claritypharmaceuticals.com/news/fap/
- Clarity Pharmaceuticals. Clarity expands pipeline in breast cancer with Cu-64/67 SAR-trastuzumab: Pre-clinical data published and trastuzumab supply agreement in place. https://www.claritypharmaceuticals.com/news/cu-64-67-sar-trastuzumab/
This announcement has been authorised for release by the Executive Chairperson.
