Sydney, Australia 11 February 2025
Highlights
- Clarity renews its focus on the breast cancer market, spearheaded by 64/67Cu-SAR-trastuzumab and reinforced by its SAR-Bombesin, SARTATE and SAR-bisPSMA products in this indication.
- It is estimated that 316,950 women will be diagnosed with invasive breast cancer in 2025 in the United States (US), with 42,170 dying from the disease1.
- Human epidermal growth factor receptor 2 (HER2) is expressed in up to 20% of breast cancers and is associated with a more aggressive type of tumour and poor prognosis2.
- Combining trastuzumab with Clarity’s proprietary SAR Technology enables the development of a copper-67 (Cu-67) based radioimmunotherapy (RIT) for HER2-positive breast cancer patients.
- Pre-clinical evidence shows dose-response reduction in tumour size and prolonged survival using 67Cu-SAR-trastuzumab compared to trastuzumab alone in a HER2-positive tumour mouse model3.
- To secure supply of clinical-grade Good Manufacturing Practice (GMP) trastuzumab, Clarity signed a new Supply Agreement with EirGenix, Inc.
- Clarity has built a strong clinical trial capability in the US and Australia that can be utilised to effectively translate new pipeline products, such as SAR-trastuzumab, into the clinic.
Clarity Pharmaceuticals (ASX: CU6) (“Clarity” or “Company”), a clinical-stage radiopharmaceutical company with a mission to develop next-generation products that improve treatment outcomes for children and adults with cancer, is pleased to announce the addition of a new asset, 64/67Cu-SAR-trastuzumab, into the Targeted Copper Theranostic (TCT) portfolio. Pre-clinical data on SAR-trastuzumab has recently been published and Clarity has signed a Supply Agreement with EirGenix, Inc. (“EirGenix”) for the clinical development and future commercial supply of clinical-grade GMP trastuzumab biosimilar, EG12014. The supply enables the development of a radiolabelled product using Clarity’s SAR Technology.
Trastuzumab is an antibody that targets HER2, which is expressed in many cancers, including some types of lung, gastric and breast cancers4. This novel theranostic asset will initially focus on breast cancer and, combined with SAR-Bombesin, SARTATE and SAR-bisPSMA, bolster Clarity’s renewed focus on this important indication.
Through pioneering work in collaboration with the University of Melbourne, the trastuzumab antibody was combined with Clarity’s proprietary SAR chelator and radiolabelled with copper-64 (Cu-64 or 64Cu) for diagnostic imaging and copper-67 (Cu-67 or 67Cu), forming an RIT3. 64Cu-SAR-trastuzumab was shown to target HER2-positive cancer cells to a very high level pre-clinically. 67Cu-SAR-trastuzumab was shown to significantly reduce the growth of HER2-expressing tumours in a dose-dependent manner, as well as to improve the survival of mice treated with the product (Figure 1).
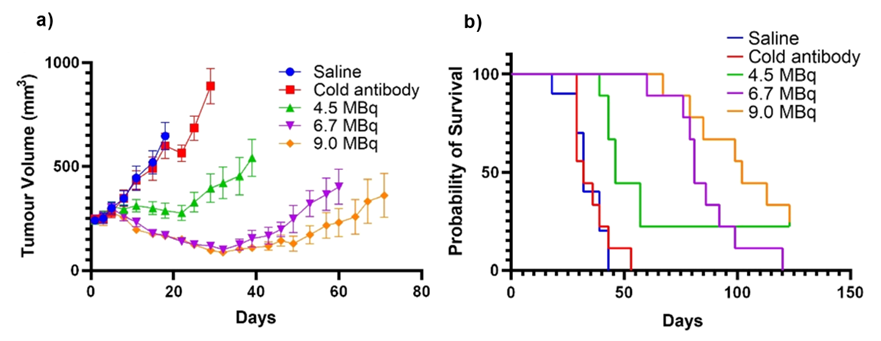
Figure 1: Treatment of HER2-positive tumours with 67Cu-SAR-trastuzumab. Pre-clinical model of HER2-expressing tumours (SKOV-3 xenograft) was used to assess the anti-tumour effect of 67Cu-SAR-trastuzumab, compared to unlabelled SAR-trastuzumab or saline (control groups). A: Treatment with a single dose of 67Cu-SAR-trastuzumab, at either 4.5, 6.7 or 9.0 MBq, inhibited tumour growth by 88%, 120% and 119% at 18 days post-administration respectively, compared to control groups (i.e. slowing of tumour growth at the 4.5 MBq dose and reducing the tumour size at higher doses at day 18). B: 67Cu-SAR-trastuzumab effectively increased the survival of all treated groups.3
Clarity intends to conduct a Phase 1/2a theranostic study with 64/67Cu-SAR-trastuzumab in HER2-positive breast cancer patients to address a significant unmet clinical need. This subtype of breast cancer is characterised as being aggressive and has a poor prognosis5. Despite recent advances in the treatment of patients with early HER2-positive breast cancer, relapse still occurs in up to 25% of patients within 10 years6.
Clarity’s Executive Chairperson, Dr Alan Taylor, commented, “The antibody revolution over the last few decades has transformed the treatment of many diseases and has also led to the development of the antibody-drug conjugate (ADC) market. However, the application of antibodies in radiopharmaceuticals, despite many potential benefits of radiation compared to the cytotoxic payloads used in ADCs, has been limited with the current strategies being sub-optimal, particularly in comparison with peptides. At Clarity, we continue to strive for excellence when it comes to science and treatment outcomes, working to firstly open up a broad range of targeting technologies for radiopharmaceuticals in a safe and effective manner, and secondly, broaden the use of radiopharmaceuticals outside of prostate cancer when looking at large cancer indications.
“We have been investigating a number of antibody strategies for many years, including the use of whole antibodies, antibody fragments and antibody pre-targeting in combination with our SAR Technology to develop a new class of treatments when combined with antibodies in RIT. The 64/67Cu-SAR-trastuzumab product is a result of this R&D process.
“The pre-clinical results we generated so far are very exciting and highlight the potential benefit of 64/67Cu-SAR-trastuzumab to image and treat patients with HER2-positive cancers. This is an area of high unmet need, as a considerable proportion of patients, most of whom are women, will relapse or develop resistance to standard treatments, and metastatic disease remains incurable. We are also committed to progressing our SAR-Bombesin, SARTATE and SAR-bisPSMA products in breast cancer, complementing the existing focus on prostate cancer, neuroblastoma and neuroendocrine tumours.
“We are uniquely placed at Clarity to address large markets, firstly with the proven background of using great science to make novel products, and secondly with the commercialisation of the “perfect pairing” of copper isotopes. Those factors combined have myriad advantages in relation to efficacy, supply and manufacturing. With regards to supply, particularly of copper-67, the simple manufacturing process on basic linear accelerators and plentiful precursor means that large markets can be served in a modular format, instead of the reliance on the small number of research reactors around the world and rare earth materials as feedstock in the case of lutetium-177 supply. The supply chains of chelatable alpha radioisotopes, that are yet to show safety and efficacy, are still in their infancy with many hurdles to overcome. Having abundant supply of diagnostic and therapeutic isotopes and finished products made under the one roof to treat large populations of patients in the same country the isotopes and products are made in is an ideal scenario quickly becoming reality and made possible with the perfect pairing of copper.
“We are excited to have entered into the Supply Agreement for trastuzumab with EirGenix, given their excellent capabilities to produce an approved biosimilar product, and progress to the next step in our RIT program. Having a reliable and secure supply of 67Cu-SAR-trastuzumab for future trials in breast cancer is essential as we continue to build a commanding pipeline of radiopharmaceuticals in order to reach our ultimate goal of better treating children and adults with cancer.”
EirGenix’s Chairman & President, Dr. Liu, commented, “We are very pleased to be working with Clarity on this important program to develop a novel treatment option for breast cancer patients. At EirGenix we believe that RIT using copper-67 is a promising avenue for the next generation of antibody therapies, and we are thrilled to be contributing to the development of this unique product.”
About SAR Technology
Clarity’s proprietary copper-chelating technology, called “sarcophagine” or SAR Technology, has enabled the Company to advance the TCT product pipeline into a range of theranostic clinical trials that use copper-64 for diagnostic imaging and copper-67 for therapy. Clarity is currently progressing three key product areas, SAR-bisPSMA, SAR-Bombesin and SARTATE, with three theranostic and four diagnostic clinical trials with a focus on prostate cancer indications.
64Cu-SAR-trastuzumab and 67Cu-SAR-trastuzumab are unregistered products. The data outlined in this announcement has not been assessed by health authorities such as the US Food and Drug Administration (FDA) or the Therapeutic Goods Administration (TGA). Outcomes from human clinical trials may differ from pre-clinical findings. A clinical development program is currently undergoing feasibility assessment. There is no guarantee that these products will become commercially available.
About Breast Cancer
Breast cancer is the most common cancer in women in the US, excluding skin cancers, although it can also affect some men. It accounts for about 30% of all new cancer diagnoses in women each year7. In 2025 in the US, an estimated 319,750 individuals are projected to be diagnosed with invasive breast cancer, with 316,950 of them being women1. Breast cancer remains the second leading cause of cancer death in women, after lung cancer. It is estimated that 42,170 women will die from breast cancer in 2025 in the US1. The lifetime risk of a woman developing breast cancer is about 13%, or 1 in 87. Incidence rates have increased slightly in recent years, especially in women under 507. Approximately up to 20% of breast cancers are HER2-positive, meaning they have higher levels of the HER2 protein, which promotes faster growth of the cancer8.
About Clarity Pharmaceuticals
Clarity is a clinical stage radiopharmaceutical company focused on the treatment of serious diseases. The Company is a leader in innovative radiopharmaceuticals, developing Targeted Copper Theranostics based on its SAR Technology Platform for the treatment of cancers in children and adults.
About EirGenix
EirGenix upholds the mission of providing clients with high-quality and cost-effective CDMO services and biologics. All in order to enhance the wellbeing of individuals and society, and improve quality of life. EG12014 (trastuzumab) is currently marketed as EIRGASUN by EirGenix in Taiwan and partnered with Sandoz and approved as Herwenda in Europe. For more information about EirGenix and their growing portfolio, visit:
Media Contacts
Clarity Pharmaceuticals
Dr Alan Taylor
Executive Chairperson
ataylor@claritypharm.com
Sodali
Catherine Strong
Investor/Media Relations
+61 (0)406 759 268
catherine.strong@sodali.com
References
- Siegel et al. Cancer statistics, 2025. CA Cancer J Clin. 2025.
- Exman et al. HER2-positive metastatic breast cancer: a comprehensive review. Clin Adv Hematol Oncol. 2021.
- Rudd et al. Potential theranostics of breast cancer with copper-64/67 sarcophagine-trastuzumab. Chem Sci. 2025.
- Iqbal & Iqbal. Human Epidermal Growth Factor Receptor 2 (HER2) in Cancers: Overexpression and Therapeutic Implications. Mol Biol Int. 2014.
- Tapia et al. Clinical Impact of New Treatment Strategies for HER2-Positive Metastatic Breast Cancer Patients with Resistance to Classical Anti-HER Therapies. Cancers. 2023.
- O’Shaughnessy et al. Risk of Recurrence in Patients With HER2+ Early-Stage Breast Cancer: Literature Analysis of Patient and Disease Characteristics. Clin Breast Cancer. 2023.
- American Cancer Society. Key statistics for breast cancer. https://www.cancer.org/cancer/types/breast-cancer/about/how-common-is-breast-cancer.html Accessed on 5 Jan 2025.
- American Cancer Society. Breast Cancer HER2 Status. https://www.cancer.org/cancer/types/breast-cancer/understanding-a-breast-cancer-diagnosis/breast-cancer-her2-status.html Accessed on 5 Jan 2025.
This announcement has been authorised for release by the Executive Chairperson.
