Sydney, Australia 5 November 2024
Highlights
- The U.S. Centers for Medicare and Medicaid Services (CMS) has published its final rule for CY2025, which establishes a separate payment for high value radiopharmaceutical diagnostics after the expiry of transitional pass-through status.
- The final ruling is a landmark victory for the radiopharmaceutical industry, promising relative price stability and ensuring clinicians and patients can select radio-diagnostics based on the best clinical performance, as opposed to the reimbursement provided during the 3-year transitional pass-through period.
- Under the new policy, CMS will unbundle and pay separately for diagnostic radiopharmaceuticals with per-day costs exceeding US$630, eliminating financial barriers that have long hindered patient access to essential nuclear medicine diagnostic procedures.
- CMS policy adjustment increases the overall value of the radio-diagnostic market as a blockbuster product category, with a positive impact on future innovation of radiopharmaceuticals.
- Clarity’s platform of diagnostic products in clinical development, including 64Cu-SAR-bisPSMA, 64Cu-SAR-Bombesin and 64Cu-SARTATE, as well as other targets in preclinical development, can all potentially benefit from this policy.
Clarity Pharmaceuticals (ASX: CU6) (“Clarity”, “the Company”), a clinical stage radiopharmaceutical company with a mission to develop next-generation products that improve treatment outcomes for children and adults with cancer, commends CMS on its CY25 final rule on radiopharmaceutical diagnostic pricing and is pleased to celebrate this major victory for patients and the radiopharmaceutical industry.
CMS recognised that the payment for nuclear medicine tests may not adequately cover the cost of specific diagnostic radiopharmaceuticals, even when those diagnostics are the most clinically suitable options for patients. As a result, it is adjusting the reimbursement policy under the CY 2025 Medicare Outpatient Prospective Payment System (OPPS) Final Rule, which will take effect January 1, 2025. This ruling preserves separate payment for high-value radio-diagnostics with per day costs above a threshold of US$630. It will improve patient access to radio-diagnostics, eliminating financial barriers, while also ensuring relative price stability for product developers beyond the transitional pass-through reimbursement period of 3 years. This change increases and solidifies the overall value of radio-diagnostics as a blockbuster market and promotes the future innovation of next-generation diagnostic radiopharmaceutical products.
Clarity’s Executive Chairperson, Dr Alan Taylor, commented, “We are thrilled to celebrate this landmark win for patients and the radiopharmaceutical field as it brings benefits to all parties involved and helps to grow the radio-diagnostic market. Patients can now get access to the imaging products without the financial barriers that tend to restrict availability; physicians and hospitals will have a stable reimbursement scheme for administering radio-diagnostics; and drug developers, like Clarity, will benefit from a market that now has a long-term focus on innovation and best-in-class products, instead of a limited opportunity during the 3-year transitional pass-through status period.
“Importantly, this change increases competitiveness in the field, ensuring that patients and their clinicians can now choose a product that is the best for identifying disease, instead of one that is selected solely based on reimbursement considerations. We have seen our optimised bisPSMA molecule continue to generate exceptional data in multiple trials to date, including the PROPELLER1 study in the pre-prostatectomy setting and the COBRA2 trial in biochemical recurrence (BCR) of prostate cancer setting. PROPELLER was Clarity’s first trial with 64Cu-SAR-bisPSMA and demonstrated improved uptake and detected more lesions than the generic 68Ga-PSMA-11 product (Figure 1). This exciting data was then further expanded by the COBRA trial that investigated the use of 64Cu-SAR-bisPSMA with same-day (1-4 hours) and next-day (~24 hours) imaging, a feature not possible with products that use 68Ga or 18F. It demonstrates that 64Cu-SAR-bisPSMA identified more lesions on both same-day and next-day imaging, including identifying lesions months earlier, compared to standard-of-care prostate-specific membrane antigen (PSMA) positron emission tomography (PET) agents (Figure 2).
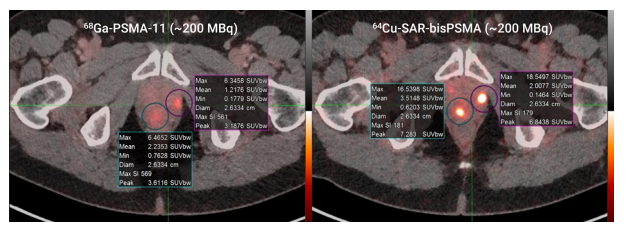
Figure 1. 68Ga-PSMA-11 (~200MBq, left image) vs. 64Cu-SAR-bisPSMA (~200MBq, right image) in the same patient. Time between serial imaging was 8 days. Standardised Uptake Value (SUVmax)* of the lesions were 6.5 and 6.3 for 68Ga-PSMA-11 and 16.5 and 18.5 for 64Cu-SAR-bisPSMA.
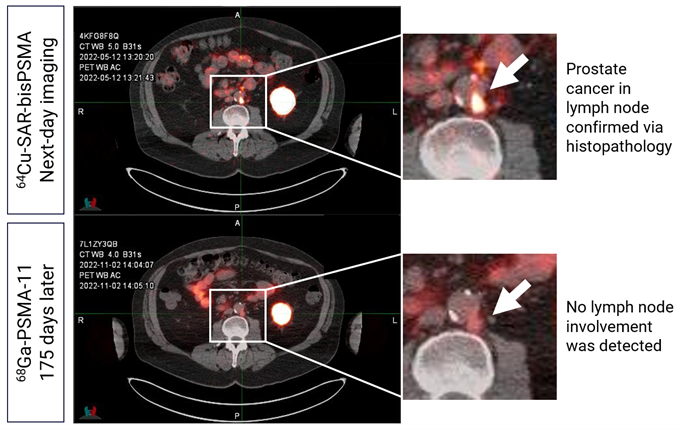
Figure 2. Retroperitoneal lesion detected by 64Cu-SAR-bisPSMA on next-day imaging. 68Ga-PSMA-11 scan performed 176 days post-Day 0 (175 days post-Day 1) did not show uptake of tracer. PET/CT fusion. Prostate cancer in lymph node confirmed via histopathology.
“With Clarity’s 2 Phase III registrational trials with 64Cu-SAR-bisPSMA, CLARIFY and AMPLIFY, progressing swiftly in these respective indications, and a Fast Track Designation recently granted by the U.S. Food and Drug Administration (FDA)3, we look forward to bringing what we believe to be the best PSMA diagnostic ever made to market and disrupting the dynamics of current-generation products, i.e. PYLARIFY, generic 68Ga-PSMA-11 (sold as Locametz and Illuccix) and POSLUMA. Not only can we deliver clinical benefits associated with increased product uptake and retention in lesions, but also improve patient access to PSMA PET imaging due to the 48-hour shelf-life of 64Cu-SAR-bisPSMA, enabled by the longer half-life of Cu-64. This means that, unlike short-lived 68Ga- and 18F-based products, we can reach patients in broader geographic areas and help eliminate disparities in cancer diagnosis around the world.
“This is an extremely exciting time for radiopharmaceuticals and the CMS ruling further highlights the importance of nuclear medicine imaging in cancer diagnosis, supporting our efforts to bring cutting-edge products to patients who need them most. We look forward to continuing to progress Clarity’s platform of radiopharmaceuticals with a number of diagnostics in development, including 64Cu-SAR-bisPSMA, 64Cu-SAR-Bombesin and 64Cu-SARTATE in the clinic, as well as many other assets as part of our pre-clinical pipeline. All these products could benefit from the CMS ruling, bringing us closer to our ultimate goal of improving treatment outcomes for children and adults with cancer.”
About Clarity Pharmaceuticals
Clarity is a clinical stage radiopharmaceutical company focused on the treatment of serious disease. The Company is a leader in innovative radiopharmaceuticals, developing targeted copper theranostics based on its SAR Technology Platform for the treatment of cancer in children and adults.
References
- Lengyelova & Emmett et al. 64Cu SAR bisPSMA (PROPELLER) Positron Emission Tomography (PET) Imaging in Patients with Confirmed Prostate Cancer. ASCO, 2023. Link or go to: https://www.claritypharmaceuticalcom/pipeline/scientific_presentations/
- Nordquist et al. COBRA: Assessment of safety and efficacy of 64Cu-SAR-bisPSMA in patients with biochemical recurrence of prostate cancer following definitive therapy. ASCO, 2024. Link or go to: https://www.claritypharmaceuticals.com/pipeline/scientific_presentations/
- Clarity Pharmaceuticals: Clarity receives FDA Fast Track Designation for 64Cu-SAR-bisPSMA, https://www.claritypharmaceuticals.com/news/fast-track/
Media Contacts
Clarity Pharmaceuticals
Dr Alan Taylor
Executive Chairperson
ataylor@claritypharm.com
Sodali
Catherine Strong
Investor/Media Relations
+61 (0)406 759 268
catherine.strong@sodali.com
This announcement has been authorised for release by the Executive Chairperson.
