Sydney, Australia 30 November 2023
Clarity Pharmaceuticals (ASX: CU6) (“Clarity”, “the Company”), a clinical stage radiopharmaceutical company with a mission to develop next-generation products that improve treatment outcomes for children and adults with cancer, is pleased to announce it has commenced its registrational Phase III 64Cu-SAR-bisPSMA diagnostic trial in prostate cancer, CLARIFY (NCT06056830)1, with the initiation of the first clinical site at the Urology Cancer Center / XCancer Omaha, NE.
The aim of the CLARIFY study is to assess the diagnostic performance of 64Cu-SAR-bisPSMA to detect regional nodal metastasis in participants with high-risk prostate cancer prior to radical prostatectomy. The study expects to recruit 383 participants at multiple clinical sites across the United States and Australia.
Evaluation will take place over 2 imaging timepoints, day 1 (day of administration) and day 2 (approximately 24 hours post administration). CLARIFY is expected to image the first participant in late 2023. As a registrational trial, the final study results are intended to provide sufficient evidence to support an application to the US FDA for approval of 64Cu-SAR-bisPSMA as a new diagnostic imaging agent in prostate cancer.
Clarity’s Executive Chairman, Dr Alan Taylor, commented, “We are excited to commence our first registrational Phase III trial with our optimised SAR-bisPSMA agent. The CLARIFY trial is based on compelling preclinical and clinical data, including our completed PROPELLER trial (NCT04839367)2,3. It demonstrated the excellent safety profile and superior performance of 64Cu-SAR-bisPSMA compared to 68Ga-PSMA-11 also in patients with prostate cancer prior to radical prostatectomy, guiding the study design for the CLARIFY trial. Despite the improvement of management and staging of patients with prostate cancer introduced by PSMA PET compared to other conventional imaging, low cancerous lesion uptake by PSMA agents may have an impact on clinical management. The PROPELLER trial showed significantly higher uptake of 64Cu-SAR-bisPSMA in PSMA-expressing lesions compared to an approved standard-of-care PSMA imaging agent, potentially enabling the diagnosis of additional and smaller lesions2,3. CLARIFY will also investigate the benefits of delayed imaging in this patient group, a feature that has shown real benefits to patients so far and is not available with the first-generation PSMA imaging agents using gallium-68 or fluorine-18, which have very short half-lives and exhibit low sensitivity in detecting cancerous lesions. Additionally, the extended shelf-life of up to 48 hours offered by 64Cu-SAR-bisPSMA not only provides greater flexibility for clinics in scheduling diagnostic scans, but also improves patient access to care, including areas with limited access to diagnostic radiopharmaceuticals in prostate cancer due to the short shelf-life of existing PSMA PET tracers.
“The prostate cancer market is a key focus area for Clarity as there is a high unmet need for diagnostics and therapy, and we believe our theranostic SAR-bisPSMA product has the potential to improve diagnosis, therapy and ultimately change patients’ lives. We look forward to progressing this trial to validate and expand upon the positive data we have accumulated so far with our SAR-bisPSMA product. With this trial, our aspiration is that with improved diagnostic tools, clinicians will be empowered to make more informed decisions regarding the best course of treatment for their patients.”
Overview of Clarity’s SAR-bisPSMA clinical trial program
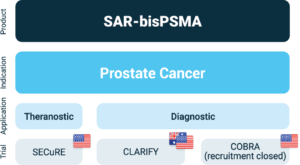
About SAR-bisPSMA
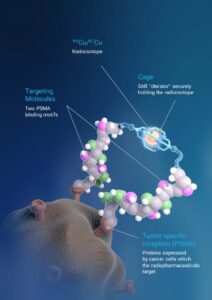
SAR-bisPSMA derives its name from the word “bis”, which reflects a novel approach of connecting two PSMA-targeting agents to Clarity’s proprietary sarcophagene (SAR) technology that securely holds copper isotopes inside a cage-like structure, called a chelator. Unlike other commercially available chelators, the SAR technology prevents copper leakage into the body. SAR-bisPSMA is a TCT that can be used with isotopes of copper-64 (Cu-64 or 64Cu) for imaging and copper-67 (Cu-67 or 67Cu) for therapy.
64Cu-SAR-bisPSMA and 67Cu-SAR-bisPSMA are unregistered products. Individual results may not represent the overall safety and efficacy of the products. The data outlined in this announcement has not been assessed by health authorities such as the US Food and Drug Administration (FDA). A clinical development program is currently underway to assess the efficacy and safety of these products. There is no guarantee that these products will become commercially available.
About Prostate Cancer
Prostate cancer is the second most common cancer diagnosed in men globally and the fifth leading cause of cancer death worldwide4. The American Cancer Institute estimates in 2023 there will be 288,300 new cases of prostate cancer in the US and around 34,700 deaths from the disease5.
About Clarity Pharmaceuticals
Clarity is a clinical stage radiopharmaceutical company focused on the treatment of serious disease. The Company is a leader in innovative radiopharmaceuticals, developing targeted copper theranostics based on its SAR Technology Platform for the treatment of cancer in children and adults.
References
- ClinicalTrials.gov Identifier: NCT06056830, https://clinicaltrials.gov/ct2/show/NCT06056830
- ClinicalTrials.gov Identifier: NCT04839367, https://clinicaltrials.gov/ct2/show/NCT04839367
- Lengyelova & Emmett et al. 64Cu-SAR-bisPSMA (PROPELLER) positron emission tomography (PET) imaging in patients with confirmed prostate cancer. ASCO 2023. Poster available at: https://www.claritypharmaceuticals.com/pipeline/scientific_presentations/
- Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries, https://acsjournals.onlinelibrary.wiley.com/doi/10.3322/caac.21660
- American Cancer Society: Key Statistics for Prostate Cancer, https://www.cancer.org/cancer/prostate-cancer/about/key-statistics.html
Media Contacts
Clarity Pharmaceuticals
Dr Alan Taylor
Executive Chairperson
+61 (0)413 871 165
ataylor@claritypharm.com
Citadel-MAGNUS
Catherine Strong
Investor/Media Relations
+61 (0)406 759 268
cstrong@citadelmagnus.com
This announcement has been authorised for release by the Executive Chairperson.
