

Sydney, Australia 27 June 2023
Highlights
- NorthStar is now producing high activity, high specific activity and high purity copper-67 (Cu-67 or 67Cu) suitable for Clarity’s ongoing clinical programs.
- Initial shipments by NorthStar for Clarity’s Targeted Copper Theranostic (TCT) clinical programs were completed under the exclusive supply agreement.
- Large-scale production of Cu-67 uses a highly efficient, environmentally preferable electron accelerator technology to meet Clarity’s growing demand for this important therapeutic radioisotope.
- Clarity’s SAR Technology enables the use of copper isotopes in radiopharmaceuticals and is the global leader in Cu-67 based therapy and Cu-64 based diagnostics.
- NorthStar is the first operational commercial-scale supplier of Cu-67 that will be exclusively supplied to Clarity for its clinical and commercial development of Cu-67 based therapy products.
BELOIT, Wis., June 26, 2023 – NorthStar Medical Radioisotopes, LLC (“NorthStar”), a global innovator in the development, production and commercialization of radiopharmaceuticals used for therapeutic and medical imaging applications, and Clarity Pharmaceuticals (“Clarity”), a clinical stage radiopharmaceutical company with a mission to develop next-generation products that improve treatment outcomes for children and adults with cancer, are pleased to announce the achievement of key milestones in the Cu-67 therapeutic radioisotope production program.
NorthStar has successfully produced high activity, high specific activity and high purity Cu-67 and has supplied the isotope for use in Clarity’s clinical programs as part of an agreement for exclusive supply of the radioisotope to Clarity. It will use NorthStar-produced Cu-67 for its three active and recruiting theranostic clinical trials evaluating the safety and efficacy of 67Cu SAR-bisPSMA, 67Cu SAR-Bombesin and 67Cu SARTATE, as well as for the future commercial rollout of TCT products. NorthStar is the first operational commercial-scale supplier of this important therapeutic radioisotope.
NorthStar’s production of Cu-67 uses a highly efficient, environmentally preferable electron accelerator technology and the radioisotope has proven suitable for radiolabeling with Clarity’s proprietary SAR Technology platform. The platform is enabled by the unique SAR chelators, specialized cage-like structures that securely hold radioisotopes of copper, and unlike traditional chelators, prevent leakage of copper isotopes into the body. Once securely attached to a targeting molecule, the cage with copper-67 is used to deliver therapeutic doses of radiation to destroy cancer cells in a targeted manner with limited damage to healthy tissue.
Clarity’s Executive Chairman, Dr Alan Taylor, commented, “We are really excited about the large-scale manufacture of Cu-67, and the timing could not be better as we continue to recruit at increasingly higher activities of Cu-67 across all three of our therapeutic clinical trials. In addition, we also continue to support clinicians’ ongoing requests for additional therapy doses for patients under the US Food and Drug Administration (FDA) Expanded Access Program (EAP). The benefits of using copper radioisotopes in radiopharmaceuticals have been discussed in the scientific literature for many years, and the combination of copper-64 for imaging and copper-67 for therapy has been dubbed the perfect pairing in the field of theranostics as both radioisotopes are the same element. However, their use has historically been hampered due to a lack of chelating technology that could hold the radioisotopes of copper inside and prevent their leakage in vivo. Clarity’s SAR Technology has made the use of TCTs possible, and we continue expanding our products into late-phase clinical trials and eventual commercial phase as we confirm the many benefits of the perfect pairing.
“Most recently, cohort 1 of our SECuRE trial with Clarity’s optimized SAR-bisPSMA product was completed in participants with metastatic castrate-resistant prostate cancer (mCRPC) who received therapy with 67Cu SAR-bisPSMA at the lowest dose level of 4GBq. Additional therapy cycles of 67Cu SAR-bisPSMA have been requested by clinicians under the EAP and early data indicates positive effects of the lowest dose of 67Cu SAR-bisPSMA on lesions, demonstrated by SPECT-CT images and a reduction in the patient’s Prostate Specific Antigen (PSA) levels.
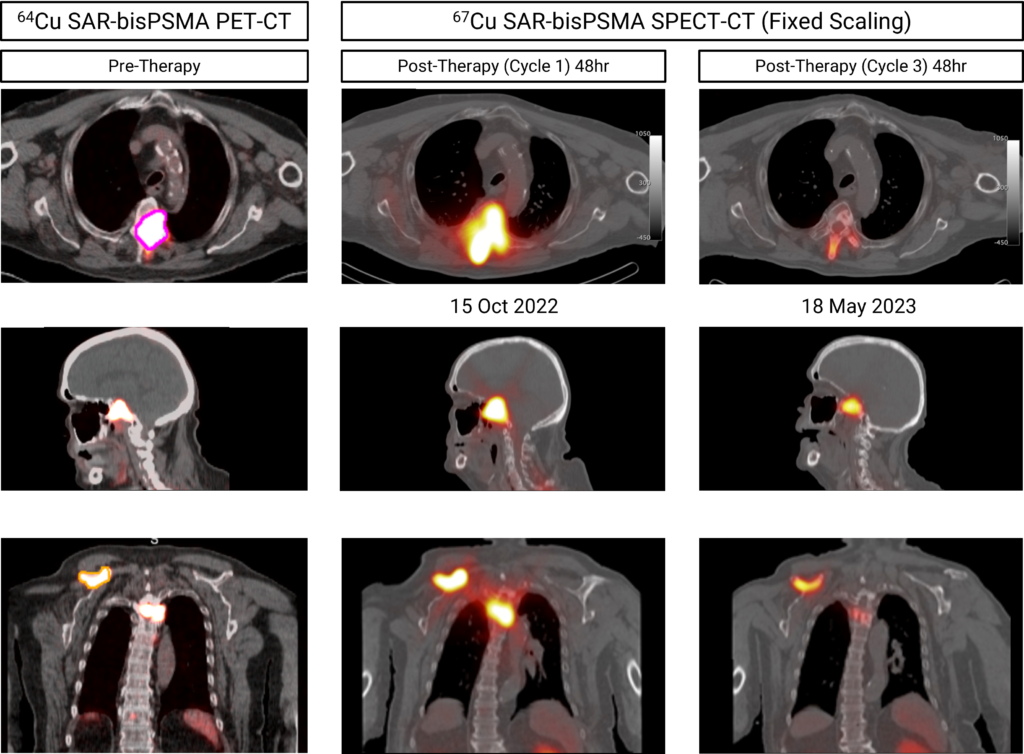
“The timely manufacture of commercial-scale, high specific activity Cu-67 complements our development with end-to-end production of radioisotope through to finished product, all in the largest oncology market in the world, the US. This puts Clarity in a unique position amongst our peers as we take full advantage of the many supply and manufacturing advantages associated with TCTs. Our paradigm of the perfect pairing lends itself to a sustainable future for radiopharmaceuticals, unhindered by the manufacturing and logistical issues currently plaguing the broader radiopharmaceuticals market. We believe that TCTs present an efficacious, scalable, sustainable and cost-effective way to expand radiopharmaceuticals into the large, global oncology market and we look forward to continuing our partnership with NorthStar as we realize our shared goal of improving treatment outcomes for children and adults with cancer,” commented Dr Taylor.
NorthStar Medical Radioisotopes’ President and Chief Executive Officer, Frank Scholz, Ph.D., commented, “This outstanding achievement of producing Cu-67 using electron accelerators is another significant demonstration of how NorthStar continues to drive commercial-scale innovation solutions for industry to meet the needs of patients and advance clinical research. Our efforts use the proven expertise and innovative approach demonstrated in the successful development and commercialization of other non-uranium produced radioisotopes in the US. We look forward to supporting Clarity’s plans for further clinical development and commercialization of Cu-67-based therapeutic radiopharmaceuticals to improve the lives of patients with serious disease.”
About Copper-67 (Cu-67)
Copper-67 (Cu-67) is an optimal-range, beta-emitting radioisotope that can be produced at commercial scale with high specific activity and without long-lived radioactive impurities. Cu-67 delivers cancer killing radiation to target cells and is being investigated for therapeutic purposes across a wide range of adult and childhood cancers, including prostate and breast cancers.
A chelator, which strongly binds Cu-67 to the targeting agent and prevents its leakage in vivo, is required to develop safe and effective targeted therapies. Clarity has successfully developed a highly specific and highly stable chelator for copper isotopes and is progressing clinical trials of a range of radiopharmaceutical products based on its proprietary SAR Technology Platform. NorthStar is rapidly advancing its proprietary process for commercial-scale production of Cu-67 to meet demand for clinical research and eventual commercial supply of TCTs.
About NorthStar Medical Radioisotopes, LLC
NorthStar Medical Radioisotopes is a commercial-stage nuclear medicine company focused on advancing patient care by providing diagnostic and therapeutic radioisotopes, novel radiopharmaceuticals and customized radiopharmaceutical development services. Its proven management team and state-of-the-art, environmentally preferable and non-uranium based technologies have made it an emerging leader at the forefront of U.S. medical radioisotope and radiopharmaceutical production. NorthStar’s molybdenum-99 (Mo-99) program is the sole source of domestic Mo-99, used to generate the standard-of-care diagnostic imaging radioisotope for assessing heart disease and cancer. It is expanding its industry-leading position in the growing area of therapeutic radioisotopes, used in targeted radiopharmaceutical therapy to treat cancer and other serious diseases, and is poised to be the first commercial-scale producer of non-carrier added (n.c.a.) actinium-225 (Ac-225) and copper-67 (Cu-67). NorthStar’s Radiopharmaceutical Contract Development and Manufacturing Organization (CDMO/CMO) services unit will provide customized service offerings and specialized radiopharmaceutical expertise to help biopharmaceutical companies rapidly advance their development and commercialization programs. For more information about NorthStar’s comprehensive portfolio and patient-focused services, visit: www.northstarnm.com.
About Clarity
Clarity is a clinical stage radiopharmaceutical company focused on the treatment of serious disease. The Company is a leader in innovative radiopharmaceuticals, developing targeted copper theranostics based on its SAR Technology Platform for the treatment of cancer in children and adults.
www.claritypharmaceuticals.com
Media Contact
For NorthStar Medical Radioisotopes, LLC
Corporate
Lisa Holst
Vice President Sales and Marketing
678-471-9027
lholst@northstarnm.com
Investor Relations
Paul Estrem
Executive Vice President and Chief Financial Officer
608-987-8318
pestrem@northstarnm.com
Media
Priscilla Harlan
781-799-7917
pharlan@shiningrockllc.com
For Clarity Pharmaceuticals
Clarity Pharmaceuticals
Dr Alan Taylor
Executive Chairman
+61 (0)413 871 165
ataylor@claritypharm.com
Citadel-MAGNUS
Catherine Strong
Investor/Media Relations
+61 (0)406 759 268
cstrong@citadelmagnus.com
This announcement has been authorised for release by the Executive Chairman.
