Sydney, Australia 14 February 2023
Highlights
- PROPELLER trial achieved all primary and secondary objectives
- 64Cu SAR-bisPSMA was safe and well tolerated by trial participants
- Uptake of Clarity’s 64Cu SAR-bisPSMA in cancer lesions is higher compared to the approved standard-of-care for prostate cancer imaging in Australia and the US, 68Ga PSMA-11
- Greater uptake into cancer lesions with 64Cu SAR-bisPSMA may enable diagnosis of additional and smaller lesions
- All product for the trial was produced centrally, eliminating the need for expensive generators and specialised personnel on or near site
- Preparations for Phase III trial underway
Clarity Pharmaceuticals (ASX: CU6) (“Clarity”), a clinical-stage radiopharmaceutical company with a mission to develop next-generation products that improve treatment outcomes for children and adults with cancer, is pleased to announce favourable imaging data from its Phase I diagnostic trial of 64Cu SAR-bisPSMA in prostate cancer, PROPELLER (NCT 048393671)1. This follows the announcement of top line data in December 2022.
This data is currently being presented via a poster at the American Society of Clinical Oncology (ASCO) Genitourinary (GU) Symposium in San Francisco. The poster will be available on Clarity’s website once released at ASCO GU.
The PROPELLER trial evaluated 30 patients with confirmed prostate cancer prior to undergoing radical prostatectomy (surgical removal of the prostate) and lymph node dissection (removal). In addition to the primary (safety, tolerability, imaging efficacy) and secondary (determining the optimal dose for subsequent investigation) endpoints, the study also compared the diagnostic properties of Clarity’s 64Cu SAR-bisPSMA product to 68Ga PSMA-11, an approved standard-of-care (SOC) product for prostate cancer imaging in Australia and the US, as an exploratory objective.
The comparison evaluated prostate cancer detection and the intensity of product uptake within the same lesions (the higher the uptake, the brighter and more visible the lesion appears on the scan). The uptake of the products was measured by maximum Standardised Uptake Values (SUVmax) on the PET scans. Importantly, all scans were evaluated by two independent, blinded, central readers.
Clarity’s Executive Chairman, Dr Alan Taylor, commented, “64Cu SAR-bisPSMA detected prostate cancer lesions that are more defined and brighter on the scans than the current SOC product, 68Ga PSMA-11. This may enable detection of smaller lesions that would have otherwise gone undetected. Arming clinicians with more accurate diagnostic information helps them determine the best course of treatment for their patients and may sometimes make the difference between the removal of the prostate, a severe and life-changing surgery, and other options that may be more effective in treating the patient’s cancer while enabling better quality of life post treatment. This patient-centric approach, reinforced by the flexibility in the timing of the scan, centralised product manufacture and its broad distribution to any imaging centre with a PET camera, would be a true paradigm shift in the management of prostate cancer. We are continuing to work diligently towards the start of the diagnostic Phase III trial of SAR-bisPSMA in the US in order to make this product available to the patients who need it most.”
Prof Louise Emmett, (St Vincent’s Hospital Sydney), Principal Investigator in the PROPELLER trial, commented, “It is very encouraging to see such positive imaging results from 64Cu SAR-bisPSMA, an agent we are very pleased to work with. The higher SUVmax was consistent across both independent, blinded, central readers, and, importantly, as shown on the images on the poster, 64Cu SAR-bisPSMA was able to detect disease outside of the primary lesion that was not detected with 68Ga PSMA-11. This is a very important result when it comes to patient management, and we are looking forward to further exploring these findings as we aim to better understand the benefits of 64Cu SAR-bisPSMA during the Phase III trial. With the high uptake of the product in tumours as well as the additional benefits of later imaging timepoints, we will continue to be involved in SAR-bisPSMA trials with the ultimate purpose of improving outcomes for our patients.”
Results
64Cu SAR-bisPSMA was shown to be safe and well tolerated across all patients, with only 1 patient in 30 reporting a metallic taste that was mild in nature (Grade 1).
A dose of 200 MBq was determined to be the optimal dose compared to other dose levels. All trials with 64Cu SAR-bisPSMA, both currently and in the future, will be undertaken at this dose level.
64Cu SAR-bisPSMA reported higher SUVmax values compared to 68Ga PSMA-11 in the 200 MBq dose cohort (n=18), according to both readers. Images from two patients comparing the 64Cu SAR-bisPSMA and 68Ga PSMA-11 scans are depicted below in Figure 1.
In this cohort, Reader 1 was able to detect primary prostate cancer in 100% of patients when 64Cu SAR-bisPSMA was used, while 68Ga PSMA-11 showed the cancer in 77.8% of patients. Similarly, Reader 2 detected primary prostate cancer in 85.7% of patients using 64Cu SAR-bisPSMA and in 83.3% of patients when using 68Ga PSMA-11.
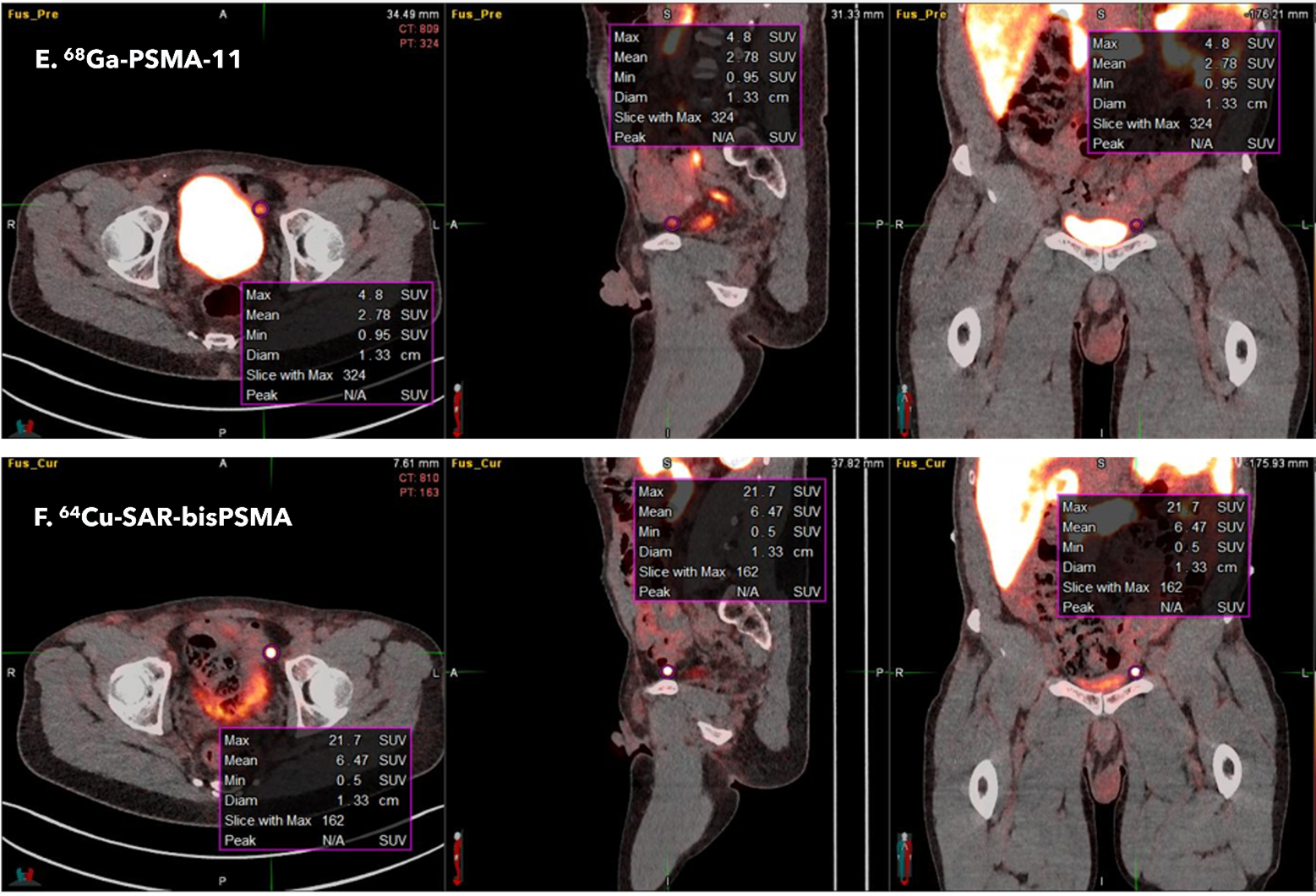
In one patient, secondary disease was detected by 64Cu SAR-bisPSMA in a pelvic lymph node that was not detected by 68Ga PSMA-11 (see Figure 2). The lymph node was subsequently determined to be positive for prostate cancer by histopathology.
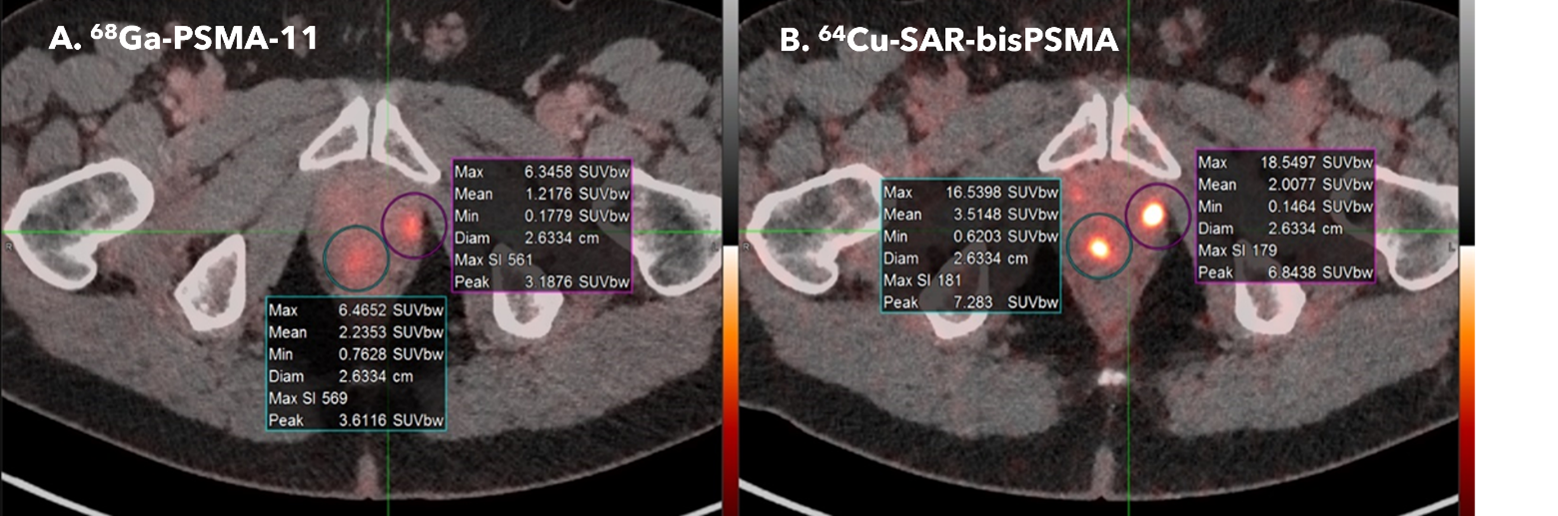
Figure 1. Scans from two patients, comparing 68Ga PSMA-11 (images A and C to the right) to 64Cu SAR-bisPSMA (images B and D to the left). In both patients, the 64Cu SAR-bisPSMA had higher uptake, leading to brighter lesions.
Patient 1 – Interval between the 68Ga PSMA-11 and 64Cu SAR-bisPSMA scan was 8 days
Patient 2 – Interval between the 68Ga PSMA-11 and 64Cu SAR-bisPSMA scan was 34 days
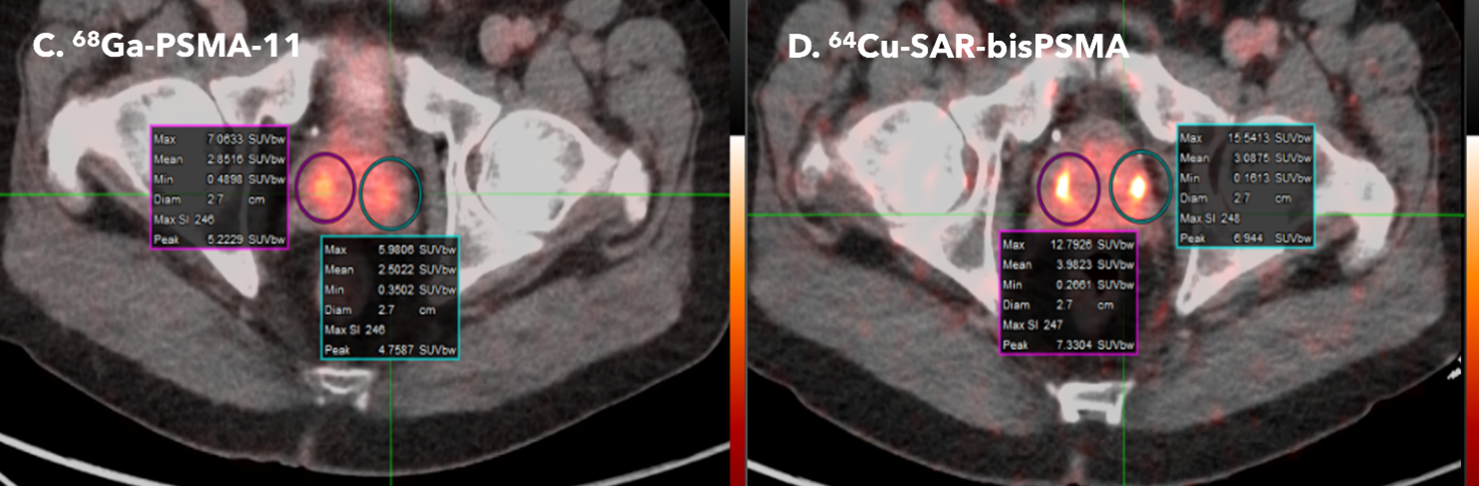
Figure 2. Scans from a third patient show uptake of 64Cu SAR-bisPSMA in a pelvic lymph node (image F in the bottom row), outside of the prostate. This lesion was not detected by the readers on the 68Ga PSMA-11 scan (image E in the top row).
Patient 3 – Interval between the 68Ga PSMA-11 and 64Cu SAR-bisPSMA scan was 7 days
Next Steps
Clarity has commenced preparations for a diagnostic Phase III trial with 64Cu SAR-bisPSMA in patients in the pre-prostatectomy/pre-definitive treatment setting. In addition, the Company is continuing the development of SAR-bisPSMA as a theranostic pair in the SECuRE trial (NCT04868604)2 and has recently met the recruitment goal of 50 patients in the COBRA trial (NCT05249127)3, investigating 64Cu SAR-bisPSMA in patients with biochemical recurrence of prostate cancer following definitive therapy.
About SAR-bisPSMA
SAR-bisPSMA derives its name from the word “bis”, which reflects a novel approach of connecting two prostate-specific membrane antigen (PSMA) binding motifs to Clarity’s proprietary sarcophagene (SAR) technology that securely holds copper isotopes inside a cage-like structure, called a chelator. Unlike other commercially available chelators, the SAR technology prevents copper leakage into the body. SAR-bisPSMA is a Targeted Copper Theranostic (TCT) that can be used with isotopes of copper-64 (Cu-64 or 64Cu) for imaging and copper-67 (Cu-67 or 67Cu) for therapy.
About Prostate Cancer
Prostate cancer is the second most common cancer diagnosed in men globally and the fifth leading cause of cancer death worldwide4. The American Cancer Institute estimates in 2023 there will be 288,300 new cases of prostate cancer in the US and around 34,700 deaths from the disease5.
About Clarity
Clarity is a clinical stage radiopharmaceutical company focused on the treatment of serious disease. The Company is a leader in innovative radiopharmaceuticals, developing targeted copper theranostics based on its SAR Technology Platform for the treatment of cancer in children and adults.
www.claritypharmaceuticals.com
References
- ClinicalTrials.gov Identifier: NCT048393671 https://clinicaltrials.gov/ct2/show/NCT04839367
- ClinicalTrials.gov Identifier: NCT04868604 https://clinicaltrials.gov/ct2/show/NCT04868604
- ClinicalTrials.gov Identifier: NCT05249127 https://clinicaltrials.gov/ct2/show/NCT05249127
- Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries https://acsjournals.onlinelibrary.wiley.com/doi/10.3322/caac.21660
- American Cancer Society: Key Statistics for Prostate Cancer, https://www.cancer.org/cancer/prostate-cancer/about/key-statistics.html
Media Contact
Clarity Pharmaceuticals
Dr Alan Taylor
Executive Chairman
+61 (0)413 871 165
ataylor@claritypharm.com
Citadel-MAGNUS
Catherine Strong
Investor/Media Relations
+61 (0)406 759 268
cstrong@citadelmagnus.com
This announcement has been authorised for release by the Executive Chairman.
